- Record: found
- Abstract: found
- Article: found
Expected and unexpected photoreactions of 9-(10-)substituted anthracene derivatives in cucurbit[ n]uril hosts†
Read this article at
Abstract
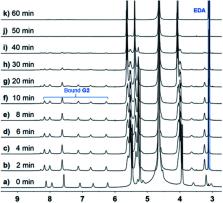
By arranging substrates in a “reaction ready” state through noncovalent interactions, supramolecular nanoreactors/catalysts show high selectivity and/or rate acceleration features. Herein, we report the host–guest complexation of 9-(10-)substituted anthracene derivatives ( G1–G3) with cucurbit[ n]uril (CB[ n], n = 8, 10), and the photoreactions of these derivatives in the presence of CB[ n] hosts. Both CB[10] and CB[8] showed no obvious effects on the photoreaction of 9,10-disubstituted derivative G1. For G2 and G3, CB[10] operated as either a nanoreactor or catalyst (10%) for the photodimerization of two compounds with high selectivity and high yield. However, although CB[8] formed a 1 : 2 complex with G2, as also observed with CB[10], the photosolvolysis product (9-anthracenemethanol) was obtained quantitatively after photoirradiation of the CB[8]·2 G2 complex. This unexpected photosolvolysis was rationalized by a plausible catalytic cycle in which anthracene acts as a photoremovable protecting group (PPG) and the carbonium ion intermediate is stabilized by CB[8].
Abstract
The photodimerization of 9-substituted anthracene derivative was tremendously promoted by a catalytic amount of cucurbit[10]uril (CB[10]) in water. While CB[8] exclusively induced the photosolvolysis of the anthracene derivative.

Related collections
Most cited references5
- Record: found
- Abstract: not found
- Book: not found