- Record: found
- Abstract: found
- Article: found
Enhanced antitumor efficacy of cisplatin in combination with HemoHIM in tumor-bearing mice
Read this article at
Abstract
Background
Although cisplatin is one of the most effective chemotherapeutic agents, cisplatin alone does not achieve a satisfactory therapeutic outcome. Also cisplatin accumulation shows toxicity to normal tissues. In this study, we examined the possibility of HemoHIM both to enhance anticancer effect with cisplatin and to reduce the side effects of cisplatin in melanoma-bearing mice.
Methods
HemoHIM was prepared by adding the ethanol-insoluble fraction to the total water extract of a mixture of 3 edible herbs, Angelica Radix, Cnidium Rhizoma and Paeonia Radix. Anticancer effects of HemoHIM with cisplatin were evaluated in melanoma-bearing mice. We used a Cr 51-release assay to measure the activity of NK/Tc cell and ELISA to evaluate the production of cytokines.
Results
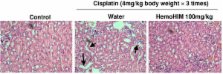
In melanoma-bearing mice, cisplatin (4 mg/kg B.W.) reduced the size and weight of the solid tumors, and HemoHIM supplementation with cisplatin enhanced the decrease of both the tumor size (p < 0.1) and weight (p < 0.1). HemoHIM itself did not inhibit melanoma cell growth in vitro, and did not disturb the effects of cisplatin in vitro. However HemoHIM administration enhanced both NK cell and Tc cell activity in mice. Interestingly, HemoHIM increased the proportion of NK cells in the spleen. In melanoma-bearing mice treated with cisplatin, HemoHIM administration also increased the activity of NK cells and Tc cells and the IL-2 and IFN-γ secretion from splenocytes, which seemed to contribute to the enhanced efficacy of cisplatin by HemoHIM. Also, HemoHIM reduced nephrotoxicity as seen by tubular cell of kidney destruction.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity.
- Record: found
- Abstract: found
- Article: not found
Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice.
- Record: found
- Abstract: found
- Article: not found