- Record: found
- Abstract: found
- Article: not found
Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies
Read this article at
Abstract
Introduction
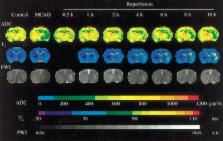
Restoration of blood flow following ischemic stroke can be achieved by means of thrombolysis or mechanical recanalization. However, for some patients, reperfusion may exacerbate the injury initially caused by ischemia, producing a so-called “cerebral reperfusion injury”. Multiple pathological processes are involved in this injury, including leukocyte infiltration, platelet and complement activation, postischemic hyperperfusion, and breakdown of the blood–brain barrier.
Methods/results and conclusions
Magnetic resonance imaging (MRI) can provide extensive information on this process of injury, and may have a role in the future in stratifying patients’ risk for reperfusion injury following recanalization. Moreover, different MRI modalities can be used to investigate the various mechanisms of reperfusion injury. Antileukocyte antibodies, brain cooling and conditioned blood reperfusion are potential therapeutic strategies for lessening or eliminating reperfusion injury, and interventionalists may play a role in the future in using some of these therapies in combination with thrombolysis or embolectomy. The present review summarizes the mechanisms of reperfusion injury and focuses on the way each of those mechanisms can be evaluated by different MRI modalities. The potential therapeutic strategies are also discussed.
Related collections
Most cited references88
- Record: found
- Abstract: found
- Article: not found
Pathophysiology of ischaemia-reperfusion injury.
- Record: found
- Abstract: found
- Article: not found
Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial.
- Record: found
- Abstract: found
- Article: not found
