- Record: found
- Abstract: found
- Article: found
Correlation between Antioxidant Activities and Phenolic Contents of Radix Angelicae Sinensis (Danggui)
Read this article at
Abstract
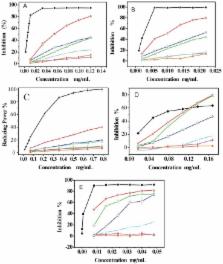
Radix Angelicae Sinensisis (RAS) is one of the most popular traditional Chinese herbal medicines. In the present study, six RAS extracts ( i.e., phenolic extract PE, petroleum ether extract PEE, ethyl acetate extract EAE, absolute ethanol extract AEE, 95% ethanol extract 95 EE, and water extract WE) were prepared and their antioxidant activities measured by DPPH (1,1-diphenyl-2-picrylhydrazyl radical), ABTS [2,2′-azino-bis(3- ethylbenzothiazoline-6-sulfonic acid diammonium salt)], Reducing power, •O 2– and lipid peroxidation assays. In general, PE, PEE and EAE had relatively high antioxidant activity, followed by AEE with moderate activity, as compared with 95 EE and WE that had low activity. Their phenolic contents (including total phenolic, ferulic acid, caffeic acid, same as below) were then determined by HPLC or spectrophotometry. The sequence of phenolic contents was roughly identical with that of antioxidant activity. When the values of 1/IC 50 of various antioxidant assays were used to evaluate the level of antioxidant of the RAS extracts, (plot between 1/IC 50 values and phenolic contents), the correlation coefficient (R) ranged from 0.642 to 0.941, with an average value of 0.839. Significant positive correlations demonstrated that the antioxidant effects of RAS might generally be considered a result of the presence of the phenolic compounds, especially ferulic acid and caffeic acid.
Related collections
Most cited references18
- Record: found
- Abstract: not found
- Article: not found
Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts
- Record: found
- Abstract: found
- Article: not found