- Record: found
- Abstract: found
- Article: found
Oxidative Stress Induces Caveolin 1 Degradation and Impairs Caveolae Functions in Skeletal Muscle Cells
Read this article at
Abstract
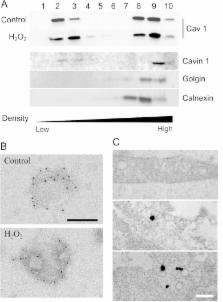
Increased level of oxidative stress, a major actor of cellular aging, impairs the regenerative capacity of skeletal muscle and leads to the reduction in the number and size of muscle fibers causing sarcopenia. Caveolin 1 is the major component of caveolae, small membrane invaginations involved in signaling and endocytic trafficking. Their role has recently expanded to mechanosensing and to the regulation of oxidative stress-induced pathways. Here, we increased the amount of reactive oxidative species in myoblasts by addition of hydrogen peroxide (H 2O 2) at non-toxic concentrations. The expression level of caveolin 1 was significantly decreased as early as 10 min after 500 μM H 2O 2 treatment. This reduction was not observed in the presence of a proteasome inhibitor, suggesting that caveolin 1 was rapidly degraded by the proteasome. In spite of caveolin 1 decrease, caveolae were still able to assemble at the plasma membrane. Their functions however were significantly perturbed by oxidative stress. Endocytosis of a ceramide analog monitored by flow cytometry was significantly diminished after H 2O 2 treatment, indicating that oxidative stress impaired its selective internalization via caveolae. The contribution of caveolae to the plasma membrane reservoir has been monitored after osmotic cell swelling. H 2O 2 treatment increased membrane fragility revealing that treated cells were more sensitive to an acute mechanical stress. Altogether, our results indicate that H 2O 2 decreased caveolin 1 expression and impaired caveolae functions. These data give new insights on age-related deficiencies in skeletal muscle.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Caveolae as plasma membrane sensors, protectors and organizers.
- Record: found
- Abstract: found
- Article: not found
Cells respond to mechanical stress by rapid disassembly of caveolae.
- Record: found
- Abstract: found
- Article: not found