- Record: found
- Abstract: found
- Article: found
Assessment of glycan interactions of clinical and avian isolates of Campylobacter jejuni
Read this article at
Abstract
Background
Campylobacter jejuni strain 11168 was demonstrated to have a broad specificity for eukaryotic surface glycosylation using glycan array analysis. The initial screen indicated that sialic acid and mannose are important binding partners after environmental stress, while galactose and fucose structures are likely to be involved in persistent infection.
Results
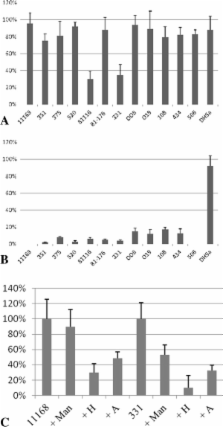
In this broader study, five additional human/clinical isolates and six chicken isolates were fully assessed to determine their glycan binding capacity using an extended glycan array. C. jejuni 11168 was rescreened here due to the presence of glycoaminoglycan (GAG) and other structures that were not available on our previous glycan array. The current array analysis of additional C. jejuni strains confirmed the growth condition dependent differences in glycan binding that was previously observed for C. jejuni 11168. We noted strain to strain variations, particularly for the human isolates C. jejuni 520 and 81116 and the chicken isolate C. jejuni 331, with the majority of differences observed in galactose, mannose and GAG binding. Chicken isolates were found to bind to a broader range of glycans compared to the human isolates, recognising branched mannose and carageenan (red seaweed) glycans. Glycan array data was confirmed using cell-based lectin inhibition assays with the fucose (UEA-I) and mannose (ConA) binding lectins.
Conclusions
This study confirms that all C. jejuni strains tested bind to a broad range of glycans, with the majority of strains (all except 81116) altering recognition of sialic acid and mannose after environmental stress. Galactose and fucose structures were bound best by all strains when C. jejuni was grown under host like conditions confirming the likelihood of these structures being involved in persistent infection.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation.
- Record: found
- Abstract: found
- Article: not found
MUC1 cell surface mucin is a critical element of the mucosal barrier to infection.
- Record: found
- Abstract: found
- Article: not found