- Record: found
- Abstract: found
- Article: found
Pasireotide Versus Octreotide in Acromegaly: A Head-to-Head Superiority Study
Read this article at
Abstract
Context:
Biochemical control reduces morbidity and increases life expectancy in patients with acromegaly. With current medical therapies, including the gold standard octreotide long-acting-release (LAR), many patients do not achieve biochemical control.
Objective:
Our objective was to demonstrate the superiority of pasireotide LAR over octreotide LAR in medically naive patients with acromegaly.
Design and Setting:
We conducted a prospective, randomized, double-blind study at 84 sites in 27 countries.
Patients:
A total of 358 patients with medically naive acromegaly (GH >5 μg/L or GH nadir ≥1 μg/L after an oral glucose tolerance test (OGTT) and IGF-1 above the upper limit of normal) were enrolled. Patients either had previous pituitary surgery but no medical treatment or were de novo with a visible pituitary adenoma on magnetic resonance imaging.
Interventions:
Patients received pasireotide LAR 40 mg/28 days (n = 176) or octreotide LAR 20 mg/28 days (n = 182) for 12 months. At months 3 and 7, titration to pasireotide LAR 60 mg or octreotide LAR 30 mg was permitted, but not mandatory, if GH ≥2.5μg/L and/or IGF-1 was above the upper limit of normal.
Main Outcome Measure:
The main outcome measure was the proportion of patients in each treatment arm with biochemical control (GH <2.5 μg/L and normal IGF-1) at month 12.
Results:
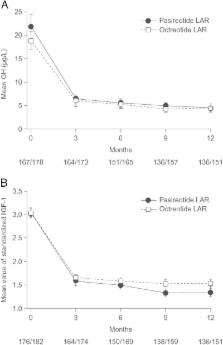
Biochemical control was achieved by significantly more pasireotide LAR patients than octreotide LAR patients (31.3% vs 19.2%; P = .007; 35.8% vs 20.9% when including patients with IGF-1 below the lower normal limit). In pasireotide LAR and octreotide LAR patients, respectively, 38.6% and 23.6% ( P = .002) achieved normal IGF-1, and 48.3% and 51.6% achieved GH <2.5 μg/L. 31.0% of pasireotide LAR and 22.2% of octreotide LAR patients who did not achieve biochemical control did not receive the recommended dose increase. Hyperglycemia-related adverse events were more common with pasireotide LAR (57.3% vs 21.7%).
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
A consensus on criteria for cure of acromegaly.
- Record: found
- Abstract: found
- Article: not found
Guidelines for acromegaly management: an update.
- Record: found
- Abstract: found
- Article: not found