- Record: found
- Abstract: found
- Article: not found
Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States
Read this article at
Abstract
Objectives: To evaluate the cost-effectiveness of a booster strategy in the US.
Methods: We developed a decision-analytic Markov model of COVID-19 to evaluate the cost-effectiveness of a booster strategy of Pfizer-BioNTech BNT162b2 (administered 6 months after 2 nd dose) among older adults, from a healthcare system perspective.
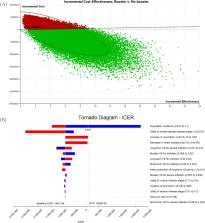
Results: Compared with 2-doses of BNT162b2 without a booster, the booster strategy in a 100,000 cohort of older adults would incur an additional cost of $3.4 million in vaccination cost, but save $6.7 million in direct medical cost and gain 3.7 QALYs in 180 days. This corresponds to a benefit-cost ratio of 1.95 and a net monetary benefit of $3.4 million. Probabilistic sensitivity analysis indicates that a booster strategy has a high chance (67%) of being cost-effective. Notably, the cost-effectiveness of the booster strategy is highly sensitive to the population incidence of COVID-19, with a cost-effectiveness threshold of 8.1/100,000 person-day. If vaccine efficacies reduce by 10%, 30%, and 50%, this threshold will increase to 9.7/100,000, 13.9/100,000, and 21.9/100,000 person-day, respectively.
Conclusion: Offering BNT162b2 booster to older adults aged ≥65 years in the US is likely to be cost-effective. Less efficacious vaccines and boosters may still be cost-effective in settings of high SARS-COV-2 transmission.
