- Record: found
- Abstract: found
- Article: found
Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells
Abstract
Purpose
Cisplatin-based chemotherapy is the first line treatment for several cancers including bladder cancer (BC). Autophagy induction has been implied to contribute to cisplatin resistance in ovarian cancer; and a high basal level of autophagy has been demonstrated in human bladder tumors. Therefore, it is reasonable to speculate that autophagy may account for the failure of cisplatin single treatment in BC. This study investigated whether cisplatin induces autophagy and the mechanism involved using human BC cell lines.
Materials and methods
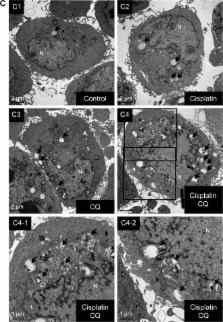
Human BC cells (5637 and T24) were used in this study. Cell viability was detected using water soluble tetrazolium-8 reagents. Autophagy induction was detected by monitoring the levels of light chain 3 (LC3)-II and p62 by Western blot, LC3-positive puncta formation by immunofluorescence, and direct observation of the autophagolysosome (AL) formation by transmission electron microscopy. Inhibitors including bafilomycin A1 (Baf A1), chloroquine (CQ), and shRNA-based lentivirus against autophagy-related genes (ATG7 and ATG12) were utilized. Apoptosis level was detected by caspase 3/7 activity and DNA fragmentation.
Results
Cisplatin decreased cell viability and induced apoptosis of 5637 and T24 cells in a dose-and time-dependent manner. The increased LC3-II accumulation, p62 clearance, the number of LC3-positive puncta, and ALs in cisplatin-treated cells suggested that cisplatin indeed induces autophagy. Inhibition of cisplatin-induced autophagy using Baf A1, CQ, or ATG7/ATG12 shRNAs significantly enhanced cytotoxicity of cisplatin toward BC cells. These results indicated that cisplatin induced protective autophagy which may contribute to the development of cisplatin resistance and resulted in treatment failure. Mechanistically, upregulation of beclin-1 (BECN1) was detected in cisplatin-treated cells, and knockdown of BECN1 using shRNA attenuated cisplatin-induced autophagy and subsequently enhanced cisplatin-induced apoptosis.
Most cited references29
- Record: found
- Abstract: found
- Article: not found
The role of autophagy in cancer development and response to therapy.
- Record: found
- Abstract: found
- Article: not found
Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase.
- Record: found
- Abstract: found
- Article: not found