- Record: found
- Abstract: found
- Article: found
The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity
Read this article at
Abstract
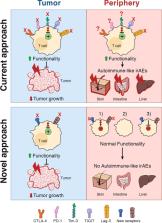
Co-inhibitory receptors are important regulators of T-cell function that define the balance between tolerance and autoimmunity. The immune regulatory function of co-inhibitory receptors, including CTLA-4, PD-1, TIM-3, TIGIT, and LAG-3, was first discovered in the setting of autoimmune disease models, in which their blockade or deficiency resulted in induction or exacerbation of the disease. Later on, co-inhibitory receptors on lymphocytes have also been found to influence outcomes in tumor and chronic viral infection settings. These receptors suppress T-cell function in the tumor microenvironment (TME), thereby making the T cells dysfunctional. Based on this observation, blockade of co-inhibitory receptors (also known as checkpoint molecules) has emerged as a successful treatment option for a number of human cancers. However, severe autoimmune-like side effects limit the use of therapeutics that block individual or combinations of co-inhibitory receptors for cancer treatment. In this review we provide an overview of the role of co-inhibitory receptors in autoimmunity and anti-tumor immunity. We then discuss current approaches and future directions to leverage our knowledge of co-inhibitory receptors to target them in tumor immunity without inducing autoimmunity.
Related collections
Most cited references171
- Record: found
- Abstract: found
- Article: not found
Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade.
- Record: found
- Abstract: found
- Article: not found
IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity.
- Record: found
- Abstract: found
- Article: not found