- Record: found
- Abstract: found
- Article: found
Beyond the Classic VTA: Extended Amygdala Projections to DA-Striatal Paths in the Primate
Read this article at
Abstract
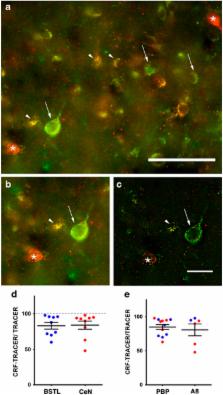
The central extended amygdala (CEA) has been conceptualized as a ‘macrosystem’ that regulates various stress-induced behaviors. Consistent with this, the CEA highly expresses corticotropin-releasing factor (CRF), an important modulator of stress responses. Stress alters goal-directed responses associated with striatal paths, including maladaptive responses such as drug seeking, social withdrawal, and compulsive behavior. CEA inputs to the midbrain dopamine (DA) system are positioned to influence striatal functions through mesolimbic DA-striatal pathways. However, the structure of this amygdala-CEA-DA neuron path to the striatum has been poorly characterized in primates. In primates, we combined neuronal tracer injections into various arms of the circuit through specific DA subpopulations to assess: (1) whether the circuit connecting amygdala, CEA, and DA cells follows CEA intrinsic organization, or a more direct topography involving bed nucleus vs central nucleus divisions; (2) CRF content of the CEA-DA path; and (3) striatal subregions specifically involved in CEA-DA-striatal loops. We found that the amygdala-CEA-DA path follows macrostructural subdivisions, with the majority of input/outputs converging in the medial central nucleus, the sublenticular extended amygdala, and the posterior lateral bed nucleus of the stria terminalis. The proportion of CRF+ outputs is >50%, and mainly targets the A10 parabrachial pigmented nucleus (PBP) and A8 (retrorubal field, RRF) neuronal subpopulations, with additional inputs to the dorsal A9 neurons. CRF-enriched CEA-DA projections are positioned to influence outputs to the ‘limbic-associative’ striatum, which is distinct from striatal regions targeted by DA cells lacking CEA input. We conclude that the concept of the CEA is supported on connectional grounds, and that CEA termination over the PBP and RRF neuronal populations can influence striatal circuits involved in associative learning.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Stress revisited: a critical evaluation of the stress concept.
- Record: found
- Abstract: found
- Article: not found
Reward representations and reward-related learning in the human brain: insights from neuroimaging.
- Record: found
- Abstract: found
- Article: not found