- Record: found
- Abstract: found
- Article: found
Large-scale identification of encystment-related proteins and genes in Pseudourostyla cristata
Read this article at
Abstract
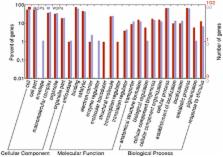
The transformation of a ciliate into cyst is an advance strategy against an adverse situation. However, the molecular mechanism for the encystation of free-living ciliates is poorly understood. A large-scale identification of the encystment-related proteins and genes in ciliate would provide us with deeper insights into the molecular mechanisms for the encystations of ciliate. We identified the encystment-related proteins and genes in Pseudourostyla cristata with shotgun LC-MS/MS and scale qRT-PCR, respectively, in this report. A total of 668 proteins were detected in the resting cysts, 102 of these proteins were high credible proteins, whereas 88 high credible proteins of the 724 total proteins were found in the vegetative cells. Compared with the vegetative cell, 6 specific proteins were found in the resting cyst. However, the majority of high credible proteins in the resting cyst and the vegetative cell were co-expressed. We compared 47 genes of the co-expressed proteins with known functions in both the cyst and the vegetative cell using scale qRT-PCR. Twenty-seven of 47 genes were differentially expressed in the cyst compared with the vegetative cell. In our identifications, many uncharacterized proteins were also found. These results will help reveal the molecular mechanism for the formation of cyst in ciliates.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Gene Ontology: tool for the unification of biology
- Record: found
- Abstract: found
- Article: not found
Large-scale analysis of the yeast proteome by multidimensional protein identification technology.
- Record: found
- Abstract: found
- Article: not found