- Record: found
- Abstract: found
- Article: found
Significance of Direct Confirmation of Growth Hormone Insensitivity for the Diagnosis of Primary IGF-I Deficiency
Read this article at
Abstract
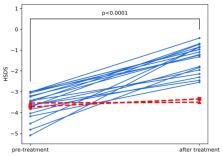
Primary insulin-like growth factor-I (IGF-I) deficiency is a synonym of growth hormone (GH) insensitivity (GHI), however the necessity of direct confirmation of GH resistance by IGF-I generation test (IGF-GT) is discussed. GHI may disturb intrauterine growth, nevertheless short children born small for gestational age (SGA) are treated with GH. We tested the hypothesis that children with appropriate birth size (AGA), height standard deviation score (SDS) <−3.0, GH peak in stimulation tests (stimGH) ≥10.0 µg/L, IGF-I <2.5 centile, and excluded GHI may benefit during GH therapy. The analysis comprised 21 AGA children compared with 6 SGA and 20 GH-deficient ones, with height SDS and IGF-I as in the studied group. All patients were treated with GH up to final height (FH). Height velocity, IGF-I, and IGF binding protein-3 (IGFBP-3) concentrations before and during first year of treatment were assessed. Effectiveness of therapy was better in GHD than in IGF-I deficiency (IGFD), with no significant difference between SGA and AGA groups. All but two AGA children responded well to GH. Pretreatment IGF-I and increase of height velocity (HV) during therapy but not the result of IGF-GT correlated with FH. As most AGA children with apparent severe IGFD benefit during GH therapy, direct confirmation of GHI seems necessary to diagnose true primary IGFD in them.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop.
- Record: found
- Abstract: not found
- Article: not found