- Record: found
- Abstract: found
- Article: found
Untargeted Metabolomic Analysis of Human Plasma Indicates Differentially Affected Polyamine and L-Arginine Metabolism in Mild Cognitive Impairment Subjects Converting to Alzheimer’s Disease
Read this article at
Abstract
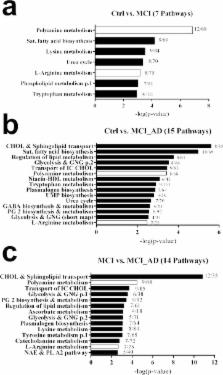
This study combined high resolution mass spectrometry (HRMS), advanced chemometrics and pathway enrichment analysis to analyse the blood metabolome of patients attending the memory clinic: cases of mild cognitive impairment (MCI; n = 16), cases of MCI who upon subsequent follow-up developed Alzheimer’s disease (MCI_AD; n = 19), and healthy age-matched controls (Ctrl; n = 37). Plasma was extracted in acetonitrile and applied to an Acquity UPLC HILIC (1.7μm x 2.1 x 100 mm) column coupled to a Xevo G2 QTof mass spectrometer using a previously optimised method. Data comprising 6751 spectral features were used to build an OPLS-DA statistical model capable of accurately distinguishing Ctrl, MCI and MCI_AD. The model accurately distinguished (R2 = 99.1%; Q2 = 97%) those MCI patients who later went on to develop AD. S-plots were used to shortlist ions of interest which were responsible for explaining the maximum amount of variation between patient groups. Metabolite database searching and pathway enrichment analysis indicated disturbances in 22 biochemical pathways, and excitingly it discovered two interlinked areas of metabolism (polyamine metabolism and L-Arginine metabolism) were differentially disrupted in this well-defined clinical cohort. The optimised untargeted HRMS methods described herein not only demonstrate that it is possible to distinguish these pathologies in human blood but also that MCI patients ‘at risk’ from AD could be predicted up to 2 years earlier than conventional clinical diagnosis. Blood-based metabolite profiling of plasma from memory clinic patients is a novel and feasible approach in improving MCI and AD diagnosis and, refining clinical trials through better patient stratification.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Global metabolic profiling procedures for urine using UPLC-MS.
- Record: found
- Abstract: found
- Article: not found
Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies.
- Record: found
- Abstract: found
- Article: not found