- Record: found
- Abstract: found
- Article: not found
Reaction of an Iron(IV) Nitrido Complex with Cyclohexadienes: Cycloaddition and Hydrogen-Atom Abstraction

Read this article at
Abstract
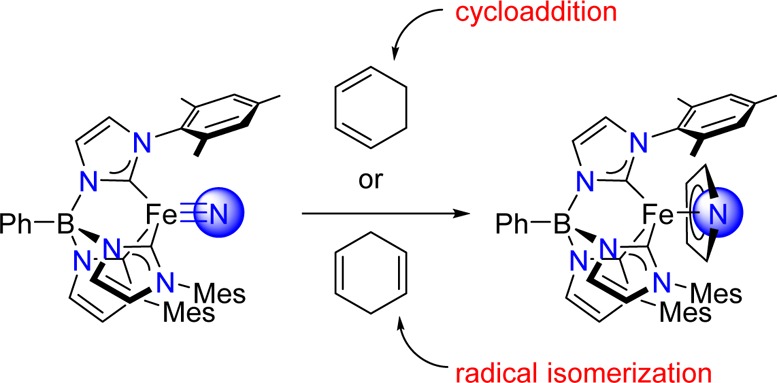
The iron(IV) nitrido complex PhB(MesIm) 3Fe≡N reacts with 1,3-cyclohexadiene to yield the iron(II) pyrrolide complex PhB(MesIm) 3Fe(η 5-C 4H 4N) in high yield. The mechanism of product formation is proposed to involve sequential [4 + 1] cycloaddition and retro Diels–Alder reactions. Surprisingly, reaction with 1,4-cyclohexadiene yields the same iron-containing product, albeit in substantially lower yield. The proposed reaction mechanism, supported by electronic structure calculations, involves hydrogen-atom abstraction from 1,4-cyclohexadiene to provide the cyclohexadienyl radical. This radical is an intermediate in substrate isomerization to 1,3-cyclohexadiene, leading to formation of the pyrrolide product.
Abstract
The iron(IV) nitrido complex PhB(MesIm) 3Fe≡N reacts with both 1,3- and 1,4-cyclohexadiene to yield the iron(II) pyrrolide complex, PhB(MesIm) 3Fe(η 5-C 4H 4N). In the case of 1,3-cyclohexadiene, product formation is proposed to occur by sequential [4 + 1] cycloaddition and retro Diels−Alder reactions. In the case of 1,4-cyclohexadiene, initial hydrogen-atom abstraction isomerizes the substrate to 1,3-cyclohexadiene, providing a pathway to the pyrrolide product.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Versatile Photocatalytic Systems for H2 Generation in Water Based on an Efficient DuBois-Type Nickel Catalyst
- Record: found
- Abstract: not found
- Article: not found
Poly(p-phenylene)-catalysed photoreduction of water to hydrogen
- Record: found
- Abstract: found
- Article: not found
