- Record: found
- Abstract: found
- Article: found
Liver-Targeting of Interferon-Alpha with Tissue-Specific Domain Antibodies
Read this article at
Abstract
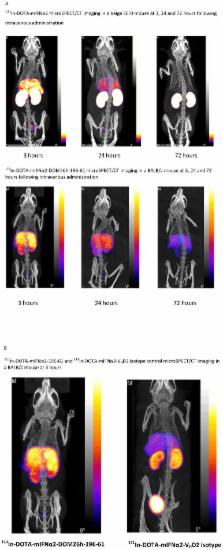
Interferon alpha (IFNα) is used for the treatment of hepatitis C infection and whilst efficacious it is associated with multiple adverse events including reduced leukocyte, erythrocyte, and platelet counts, fatigue, and depression. These events are most likely caused by systemic exposure to interferon. We therefore hypothesise that targeting the therapeutic directly to the intended site of action in the liver would reduce exposure in blood and peripheral tissue and hence improve the safety and tolerability of IFNα therapy. We genetically fused IFN to a domain antibody (dAb) specific to a hepatocyte restricted antigen, asialoglycoprotein receptor (ASGPR). Our results show that the murine IFNα2 homolog (mIFNα2) fused to an ASGPR specific dAb, termed DOM26h-196-61, could be expressed in mammalian tissue culture systems and retains the desirable biophysical properties and activity of both fusion partners when measured in vitro. Furthermore a clear increase in in vivo targeting of the liver by mIFNα2-ASGPR dAb fusion protein, compared to that observed with either unfused mIFNα2 or mIFNα2 fused to an isotype control dAb V HD2 (which does not bind ASGPR) was demonstrated using microSPECT imaging. We suggest that these findings may be applicable in the development of a liver-targeted human IFN molecule with improved safety and patient compliance in comparison to the current standard of care, which could ultimately be used as a treatment for human hepatitis virus infections.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli.
- Record: found
- Abstract: found
- Article: not found
Domain antibodies: proteins for therapy.
- Record: found
- Abstract: found
- Article: not found