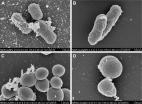
Background and aim Bimetallic silver/gold nanosystems are expected to significantly improve therapeutic efficacy compared to their monometallic counterparts by maintaining the general biocompatibility of gold nanoparticles (AuNPs) while, at the same time, decreasing the relatively high toxicity of silver nanoparticles (AgNPs) toward healthy human cells. Thus, the aim of this research was to establish a highly reproducible one-pot green synthesis of colloidal AuNPs and bimetallic Ag/Au alloy nanoparticles (NPs; Ag/AuNPs) using starch as reducing and capping agent. Methods The optical properties, high reproducibility, stability and particle size distribution of the colloidal NPs were analyzed by ultraviolet (UV)–visible spectroscopy, dynamic light scattering (DLS) and ζ-potential. The presence of starch as capping agent was determined by Fourier transform infrared (FT-IR) spectroscopy. The structural properties were studied by X-ray diffraction (XRD). Transmission electron microscopy (TEM) imaging was done to determine the morphology and size of the nanostructures. The chemical composition of the nanomaterials was determined by energy-dispersive X-ray spectroscopy (EDS) and inductively coupled plasma mass spectrometry (ICP-MS) analysis. To further study the biomedical applications of the synthesized nanostructures, antibacterial studies against multidrug-resistant (MDR) Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) were conducted. In addition, the NPs were added to the growth media of human dermal fibroblast (HDF) and human melanoma cells to show their cytocompatibility and cytotoxicity, respectively, over a 3-day experiment. Results UV–visible spectroscopy confirmed the highly reproducible green synthesis of colloidal AuNPs and Ag/AuNPs. The NPs showed a face-centered cubic crystal structure and an icosahedral shape with mean particle sizes of 28.5 and 9.7 nm for AuNPs and Ag/AuNPs, respectively. The antibacterial studies of the NPs against antibiotic-resistant bacterial strains presented a dose-dependent antimicrobial behavior. Furthermore, the NPs showed cytocompat-ibility towards HDF, but a dose-dependent anticancer effect was found when human melanoma cells were grown in presence of different NP concentrations for 72 hours. Conclusion In this study, mono- and bimetallic NPs were synthesized for the first time using a highly reproducible, environmentally friendly, cost-effective and quick method and were successfully characterized and tested for several anti-infection and anticancer biomedical applications.