- Record: found
- Abstract: found
- Article: found
The how’s and what’s of vaccine reactogenicity
Read this article at
Abstract
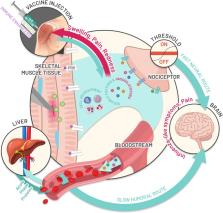
Reactogenicity represents the physical manifestation of the inflammatory response to vaccination, and can include injection-site pain, redness, swelling or induration at the injection site, as well as systemic symptoms, such as fever, myalgia, or headache. The experience of symptoms following vaccination can lead to needle fear, long-term negative attitudes and non-compliant behaviours, which undermine the public health impact of vaccination. This review presents current knowledge on the potential causes of reactogenicity, and how host characteristics, vaccine administration and composition factors can influence the development and perception of reactogenicity. The intent is to provide an overview of reactogenicity after vaccination to help the vaccine community, including healthcare professionals, in maintaining confidence in vaccines by promoting vaccination, setting expectations for vaccinees about what might occur after vaccination and reducing anxiety by managing the vaccination setting.
Related collections
Most cited references91

- Record: found
- Abstract: found
- Article: found
The State of Vaccine Confidence 2016: Global Insights Through a 67-Country Survey
- Record: found
- Abstract: found
- Article: not found
Interactions between the immune and nervous systems in pain.
- Record: found
- Abstract: found
- Article: not found