- Record: found
- Abstract: found
- Article: found
Vedolizumab for inflammatory bowel disease: From randomized controlled trials to real-life evidence
Read this article at
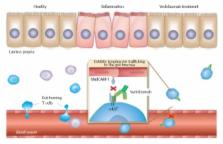
Abstract
The biologic antitumor necrosis factor alpha (anti-TNFα) agents have revolutionised the treatment of inflammatory bowel disease (IBD). However, some patients experience primary nonresponse, loss of response, or intolerance. Therefore, introducing a newer class of therapy with a mechanism of action that acts on different inflammatory pathways involved in IBD pathogenesis is appealing. Vedolizumab is a fully humanised monoclonal antibody that selectively targets α4β7 integrin. Based on the results of the pivotal clinical GEMINI trials, vedolizumab was approved for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) and Crohn’s disease (CD) refractory or intolerant to either conventional therapy or TNFα inhibitors. This review describes the efficacy, safety, and tolerability of vedolizumab reported in both randomized, controlled, clinical trials and from real-world experience in patients with UC and CD in order to identify its place in treatment algorithms for IBD.
Related collections
Most cited references45
- Record: found
- Abstract: not found
- Article: not found
A simple index of Crohn's-disease activity.
- Record: found
- Abstract: found
- Article: not found
Review article: loss of response to anti-TNF treatments in Crohn's disease.
- Record: found
- Abstract: found
- Article: not found