- Record: found
- Abstract: found
- Article: found
Economic evaluation of pneumococcal conjugate vaccination in The Gambia
Read this article at
Abstract
Background
Gambia is the second GAVI support-eligible country to introduce the 7-valent pneumococcal conjugate vaccine (PCV7), but a country-specific cost-effectiveness analysis of the vaccine is not available. Our objective was to assess the potential impact of PCVs of different valences in The Gambia.
Methods
We synthesized the best available epidemiological and cost data using a state-transition model to simulate the natural histories of various pneumococcal diseases. For the base-case, we estimated incremental cost (in 2005 US dollars) per disability-adjusted life year (DALY) averted under routine vaccination using PCV9 compared to no vaccination. We extended the base-case results for PCV9 to estimate the cost-effectiveness of PCV7, PCV10, and PCV13, each compared to no vaccination. To explore parameter uncertainty, we performed both deterministic and probabilistic sensitivity analyses. We also explored the impact of vaccine efficacy waning, herd immunity, and serotype replacement, as a part of the uncertainty analyses, by assuming alternative scenarios and extrapolating empirical results from different settings.
Results
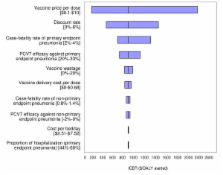
Assuming 90% coverage, a program using a 9-valent PCV (PCV9) would prevent approximately 630 hospitalizations, 40 deaths, and 1000 DALYs, over the first 5 years of life of a birth cohort. Under base-case assumptions ($3.5 per vaccine), compared to no intervention, a PCV9 vaccination program would cost $670 per DALY averted in The Gambia. The corresponding values for PCV7, PCV10, and PCV13 were $910, $670, and $570 per DALY averted, respectively. Sensitivity analyses that explored the implications of the uncertain key parameters showed that model outcomes were most sensitive to vaccine price per dose, discount rate, case-fatality rate of primary endpoint pneumonia, and vaccine efficacy against primary endpoint pneumonia.
Conclusions
Based on the information available now, infant PCV vaccination would be expected to reduce pneumococcal diseases caused by S. pneumoniae in The Gambia. Assuming a cost-effectiveness threshold of three times GDP per capita, all PCVs examined would be cost-effective at the tentative Advance Market Commitment (AMC) price of $3.5 per dose. Because the cost-effectiveness of a PCV program could be affected by potential serotype replacement or herd immunity effects that may not be known until after a large scale introduction, type-specific surveillance and iterative evaluation will be critical.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies.
- Record: found
- Abstract: found
- Article: not found
Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998-2003.
- Record: found
- Abstract: found
- Article: not found