- Record: found
- Abstract: found
- Article: found
Right Dose Right Now: bedside data-driven personalized antibiotic dosing in severe sepsis and septic shock — rationale and design of a multicenter randomized controlled superiority trial
Read this article at
Abstract
Background
Antibiotic exposure is often inadequate in critically ill patients with severe sepsis or septic shock and this is associated with worse outcomes. Despite markedly altered and rapidly changing pharmacokinetics in these patients, guidelines and clinicians continue to rely on standard dosing schemes. To address this challenge, we developed AutoKinetics, a clinical decision support system for antibiotic dosing. By feeding large amounts of electronic health record patient data into pharmacokinetic models, patient-specific predicted future plasma concentrations are displayed graphically. In addition, a tailored dosing advice is provided at the bedside in real time. To evaluate the effect of AutoKinetics on pharmacometric and clinical endpoints, we are conducting the Right Dose Right Now multicenter, randomized controlled, two-arm, parallel-group, non-blinded, superiority trial.
Methods
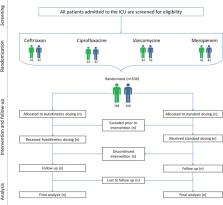
All adult intensive care patients with a suspected or proven infection and having either lactatemia or receiving vasopressor support are eligible for inclusion. Randomization to the AutoKinetics or control group is initiated at the bedside when prescribing at least one of four commonly administered antibiotics: ceftriaxone, ciprofloxacin, meropenem and vancomycin. Dosing advice is available for patients in the AutoKinetics group, whereas patients in the control group receive standard dosing.
The primary outcome of the study is pharmacometric target attainment during the first 24 h. Power analysis revealed the need for inclusion of 42 patients per group per antibiotic. Thus, a total of 336 patients will be included, 168 in each group. Secondary pharmacometric endpoints include time to target attainment and fraction of target attainment during an entire antibiotic course. Secondary clinical endpoints include mortality, clinical cure and days free from organ support. Several other exploratory and subgroup analyses are planned.
Discussion
This is the first randomized controlled trial to assess the effectiveness and safety of bedside data-driven automated antibiotic dosing advice. This is important as adequate antibiotic exposure may be crucial to treat severe sepsis and septic shock. In addition, the trial could prove to be a significant contribution to clinical pharmacometrics and serve as a stepping stone for the use of big data and artificial intelligence in the field.
Trial registration
Netherlands Trial Register (NTR), NL6501/NTR6689. Registered on 25 August 2017.
European Clinical Trials Database (EudraCT), 2017-002478-37. Registered on 6 November 2017.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: found
Epidemiology of severe sepsis
- Record: found
- Abstract: found
- Article: not found
DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients?
- Record: found
- Abstract: not found
- Article: not found