- Record: found
- Abstract: found
- Article: found
Annexin A2 Is a Natural Extrahepatic Inhibitor of the PCSK9-Induced LDL Receptor Degradation
Read this article at
Abstract
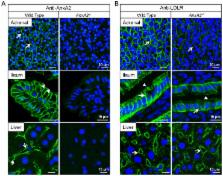
Proprotein convertase subtilisin/kexin-9 (PCSK9) enhances the degradation of hepatic low-density lipoprotein receptor (LDLR). Deletion of PCSK9, and loss-of-function mutants in humans result in lower levels of circulating LDL-cholesterol and a strong protection against coronary heart disease. Accordingly, the quest for PCSK9 inhibitors has major clinical implications. We have previously identified annexin A2 (AnxA2) as an endogenous binding partner and functional inhibitor of PCSK9. Herein, we studied the relevance of AnxA2 in PCSK9 inhibition and lipid metabolism in vivo. Plasma analyses of AnxA2 −/− mice revealed: i) a ∼1.4-fold increase in LDL-cholesterol without significant changes in VLDLs or HDLs, and ii) a ∼2-fold increase in circulating PCSK9 levels. Western blotting and immunohistochemistry of AnxA2 −/− tissues revealed that the LDLR was decreased by ∼50% in extrahepatic tissues, such as adrenals and colon. We also show that AnxA2-derived synthetic peptides block the PCSK9≡LDLR interaction in vitro, and adenoviral overexpression of AnxA2 in mouse liver increases LDLR protein levels in vivo. These results suggest that AnxA2 acts as an endogenous regulator of LDLR degradation, mostly in extrahepatic tissues. Finally, we identified an AnxA2 coding polymorphism, V98L, that correlates with lower circulating levels of PCSK9 thereby extending our results on the physiological role of AnxA2 in humans.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation.
- Record: found
- Abstract: found
- Article: not found
PCSK9: a convertase that coordinates LDL catabolism.
- Record: found
- Abstract: found
- Article: not found