- Record: found
- Abstract: found
- Article: found
Feline Immunodeficiency Virus Neuropathogenesis: A Model for HIV-Induced CNS Inflammation and Neurodegeneration
Read this article at
Abstract
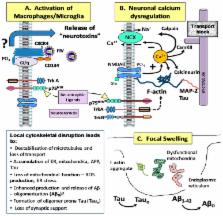
Feline Immunodeficiency virus (FIV), similar to its human analog human immunodeficiency virus (HIV), enters the central nervous system (CNS) soon after infection and establishes a protected viral reservoir. The ensuing inflammation and damage give rise to varying degrees of cognitive decline collectively known as HIV-associated neurocognitive disorders (HAND). Because of the similarities to HIV infection and disease, FIV has provided a useful model for both in vitro and in vivo studies of CNS infection, inflammation and pathology. This mini review summarizes insights gained from studies of early infection, immune cell trafficking, inflammation and the mechanisms of neuropathogenesis. Advances in our understanding of these processes have contributed to the development of therapeutic interventions designed to protect neurons and regulate inflammatory activity.
Related collections
Most cited references134
- Record: found
- Abstract: found
- Article: not found
Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus.
- Record: found
- Abstract: found
- Article: not found
Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome.
- Record: found
- Abstract: found
- Article: not found