- Record: found
- Abstract: found
- Article: found
Type I interferon induces CXCL13 to support ectopic germinal center formation

Read this article at
Abstract
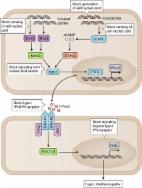
Denton et al. show that during influenza infection of mice, type I interferon can induce CXCL13 de novo in pulmonary PGDFRα + fibroblasts. This chemokine drives CXCR5-dependent recruitment of B cells to the lung, thereby supporting pulmonary germinal center formation.
Abstract
Ectopic lymphoid structures form in a wide range of inflammatory conditions, including infection, autoimmune disease, and cancer. In the context of infection, this response can be beneficial for the host: influenza A virus infection–induced pulmonary ectopic germinal centers give rise to more broadly cross-reactive antibody responses, thereby generating cross-strain protection. However, despite the ubiquity of ectopic lymphoid structures and their role in both health and disease, little is known about the mechanisms by which inflammation is able to convert a peripheral tissue into one that resembles a secondary lymphoid organ. Here, we show that type I IFN produced after viral infection can induce CXCL13 expression in a phenotypically distinct population of lung fibroblasts, driving CXCR5-dependent recruitment of B cells and initiating ectopic germinal center formation. This identifies type I IFN as a novel inducer of CXCL13, which, in combination with other stimuli, can promote lung remodeling, converting a nonlymphoid tissue into one permissive to functional tertiary lymphoid structure formation.
Graphical Abstract
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
CXCR3 in T cell function.
- Record: found
- Abstract: found
- Article: found
Type I interferon–mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview
- Record: found
- Abstract: found
- Article: not found
