- Record: found
- Abstract: found
- Article: found
Investigation of Interactions between Thrombin and Ten Phenolic Compounds by Affinity Capillary Electrophoresis and Molecular Docking
Read this article at
Abstract
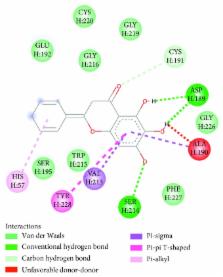
Thrombin plays a vital role in blood coagulation, which is a key process involved in thrombosis by promoting platelet aggregation and converting fibrinogen to form the fibrin clot. In the receptor concept, drugs produce their therapeutic effects via interactions with the targets. Therefore, investigation of interaction between thrombin and small molecules is important to find out the potential thrombin inhibitor. In this study, affinity capillary electrophoresis (ACE) and in silico molecular docking methods were developed to study the interaction between thrombin and ten phenolic compounds ( p-hydroxybenzoic acid, protocatechuic acid, vanillic acid, gallic acid, catechin, epicatechin, dihydroquercetin, naringenin, apigenin, and baicalein). The ACE results showed that gallic acids and six flavonoid compounds had relative strong interactions with thrombin. In addition, the docking results indicated that all of optimal conformations of the six flavonoid compounds were positioned into the thrombin activity centre and had interaction with the HIS57 or SER195 which was the key residue to bind thrombin inhibitors such as argatroban. Herein, these six flavonoid compounds might have the potential of thrombin inhibition activity. In addition, the developed method in this study can be further applied to study the interactions of other molecules with thrombin.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Drug-protein binding: a critical review of analytical tools.
- Record: found
- Abstract: found
- Article: not found
A series of natural flavonoids as thrombin inhibitors: structure-activity relationships.
- Record: found
- Abstract: found
- Article: not found