- Record: found
- Abstract: found
- Article: found
Active Trafficking of Alpha 1 Antitrypsin across the Lung Endothelium
Read this article at
Abstract
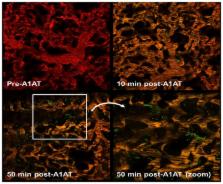
The homeostatic lung protective effects of alpha-1 antitrypsin (A1AT) may require the transport of circulating proteinase inhibitor across an intact lung endothelial barrier. We hypothesized that uninjured pulmonary endothelial cells transport A1AT to lung epithelial cells. Purified human A1AT was rapidly taken up by confluent primary rat pulmonary endothelial cell monolayers, was secreted extracellularly, both apically and basolaterally, and was taken up by adjacent rat lung epithelial cells co-cultured on polarized transwells. Similarly, polarized primary human lung epithelial cells took up basolaterally-, but not apically-supplied A1AT, followed by apical secretion. Evidence of A1AT transcytosis across lung microcirculation was confirmed in vivo by two-photon intravital microscopy in mice. Time-lapse confocal microscopy indicated that A1AT co-localized with Golgi in the endothelium whilst inhibition of the classical secretory pathway with tunicamycin significantly increased intracellular retention of A1AT. However, inhibition of Golgi secretion promoted non-classical A1AT secretion, associated with microparticle release. Polymerized A1AT or A1AT supplied to endothelial cells exposed to soluble cigarette smoke extract had decreased transcytosis. These results suggest previously unappreciated pathways of A1AT bidirectional uptake and secretion from lung endothelial cells towards the alveolar epithelium and airspaces. A1AT trafficking may determine its functional bioavailablity in the lung, which could be impaired in individuals exposed to smoking or in those with A1AT deficiency.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Functional connectivity between immune cells mediated by tunneling nanotubules.
- Record: found
- Abstract: found
- Article: not found
A New Function for the LDL Receptor: Transcytosis of LDL across the Blood–Brain Barrier
- Record: found
- Abstract: found
- Article: not found