- Record: found
- Abstract: found
- Article: found
Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients
Read this article at
Abstract
Background
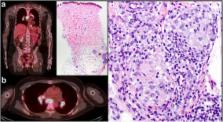
Immune checkpoint therapy has dramatically changed the landscape of cancer therapy, providing an efficacious and durable therapeutic option for patients with advanced-stage disease. However, dermatologic toxicities are a well-recognized side effect in patients receiving this therapy. A spectrum of immune related adverse events (irAEs) involving the skin can occur and include immunobullous disorders, lichenoid dermatitis, and vitiligo. Granulomatous/sarcoid-like lesions are now being recognized with the current class of checkpoint inhibitors (CPIs) that involve the dermis, the subcutaneous tissue (panniculitis), and lymph nodes.
Case presentation
We report 3 patients who developed granulomatous/sarcoid-like lesions while being treated with immune checkpoint therapy for advanced-stage melanoma, and we provide a comprehensive review of the literature in which similar cases are described. To date, 26 patients (including the 3 from this report) have been described with a median age of 57 years who developed granulomatous/sarcoid-like lesions associated with CPIs (median onset 6 months), of which 77% of patients had melanoma as primary tumor. To manage this adverse side effect, therapy was withheld in 38% of patients and 44% of the patients were treated with systemic steroids and 8% patients with localized therapy (one patient with intralesional triamcinolone). 96% of patients demonstrated either resolution or improvement of granulomatous/sarcoid-like lesions associated with CPIs irrespective of medical intervention. Therapeutic response, stable disease, or remission of primary malignancy was observed in 71% of reported patients who developed granulomatous/sarcoid-like lesions associated with CPIs over a median follow-up of 11.5 months since initiation of treatment.
Conclusions
The development of granulomatous/sarcoid-like lesions associated with CPIs is a recognized manifestation with the current class of immune checkpoint therapy that may clinically and radiographically mimic disease recurrence. Awareness of this type of toxicity is important for appropriate management and possible measurement of therapeutic response in a subset of patients who manifest this type of immune-mediated reaction.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Sarcoidosis.
- Record: found
- Abstract: found
- Article: not found
Neoantigen landscape dynamics during human melanoma-T cell interactions.
- Record: found
- Abstract: found
- Article: not found