- Record: found
- Abstract: found
- Article: not found
1H NMR Shows Slow Phospholipid Flip-Flop in Gel and Fluid Bilayers

Read this article at
Abstract

We measured the transbilayer diffusion of 1,2-dipalmitoyl- sn-glycero-3-phosphocholine (DPPC) in large unilamellar vesicles, in both the gel ( L β′) and fluid ( L α) phases. The choline resonance of headgroup-protiated DPPC exchanged into the outer leaflet of headgroup-deuterated DPPC- d13 vesicles was monitored using 1H NMR spectroscopy, coupled with the addition of a paramagnetic shift reagent. This allowed us to distinguish between the inner and outer bilayer leaflet of DPPC, to determine the flip-flop rate as a function of temperature. Flip-flop of fluid-phase DPPC exhibited Arrhenius kinetics, from which we determined an activation energy of 122 kJ mol –1. In gel-phase DPPC vesicles, flip-flop was not observed over the course of 250 h. Our findings are in contrast to previous studies of solid-supported bilayers, where the reported DPPC translocation rates are at least several orders of magnitude faster than those in vesicles at corresponding temperatures. We reconcile these differences by proposing a defect-mediated acceleration of lipid translocation in supported bilayers, where long-lived, submicron-sized holes resulting from incomplete surface coverage are the sites of rapid transbilayer movement.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: found
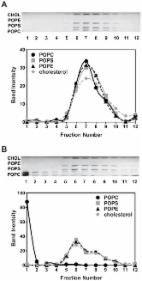
Preparation of Artificial Plasma Membrane Mimicking Vesicles with Lipid Asymmetry
- Record: found
- Abstract: found
- Article: not found
Revitalizing membrane rafts: new tools and insights.
- Record: found
- Abstract: found
- Article: not found
