- Record: found
- Abstract: found
- Article: found
Tau PET imaging in neurodegenerative tauopathies—still a challenge
Read this article at
Abstract
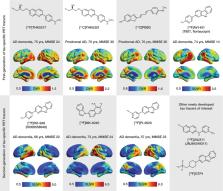
The accumulation of pathological misfolded tau is a feature common to a collective of neurodegenerative disorders known as tauopathies, of which Alzheimer’s disease (AD) is the most common. Related tauopathies include progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), Down’s syndrome (DS), Parkinson’s disease (PD), and dementia with Lewy bodies (DLB). Investigation of the role of tau pathology in the onset and progression of these disorders is now possible due the recent advent of tau-specific ligands for use with positron emission tomography (PET), including first- (e.g., [ 18F]THK5317, [ 18F]THK5351, [ 18F]AV1451, and [ 11C]PBB3) and second-generation compounds [namely [ 18F]MK-6240, [ 18F]RO-948 (previously referred to as [ 18F]RO69558948), [ 18F]PI-2620, [ 18F]GTP1, [ 18F]PM-PBB3, and [ 18F]JNJ64349311 ([ 18F]JNJ311) and its derivative [ 18F]JNJ-067)]. In this review we describe and discuss findings from in vitro and in vivo studies using both initial and new tau ligands, including their relation to biomarkers for amyloid-β and neurodegeneration, and cognitive findings. Lastly, methodological considerations for the quantification of in vivo ligand binding are addressed, along with potential future applications of tau PET, including therapeutic trials.
Related collections
Most cited references162
- Record: found
- Abstract: found
- Article: not found
Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria.
- Record: found
- Abstract: found
- Article: not found
Primary age-related tauopathy (PART): a common pathology associated with human aging.
- Record: found
- Abstract: found
- Article: not found