- Record: found
- Abstract: found
- Article: found
Development of a Three‐Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling
Read this article at
Abstract
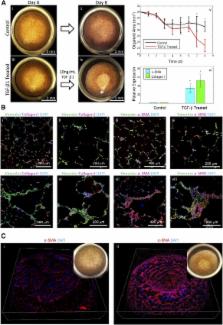
Stem cell technologies, especially patient‐specific, induced stem cell pluripotency and directed differentiation, hold great promise for changing the landscape of medical therapies. Proper exploitation of these methods may lead to personalized organ transplants, but to regenerate organs, it is necessary to develop methods for assembling differentiated cells into functional, organ‐level tissues. The generation of three‐dimensional human tissue models also holds potential for medical advances in disease modeling, as full organ functionality may not be necessary to recapitulate disease pathophysiology. This is specifically true of lung diseases where animal models often do not recapitulate human disease. Here, we present a method for the generation of self‐assembled human lung tissue and its potential for disease modeling and drug discovery for lung diseases characterized by progressive and irreversible scarring such as idiopathic pulmonary fibrosis (IPF). Tissue formation occurs because of the overlapping processes of cellular adhesion to multiple alveolar sac templates, bioreactor rotation, and cellular contraction. Addition of transforming growth factor‐β1 to single cell‐type mesenchymal organoids resulted in morphologic scarring typical of that seen in IPF but not in two‐dimensional IPF fibroblast cultures. Furthermore, this lung organoid may be modified to contain multiple lung cell types assembled into the correct anatomical location, thereby allowing cell‐cell contact and recapitulating the lung microenvironment. Our bottom‐up approach for synthesizing patient‐specific lung tissue in a scalable system allows for the development of relevant human lung disease models with the potential for high throughput drug screening to identify targeted therapies. S tem C ells T ranslational M edicine 2017;6:622–633
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy.
- Record: found
- Abstract: found
- Article: not found
Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis.
- Record: found
- Abstract: found
- Article: not found