- Record: found
- Abstract: found
- Article: found
The Long Non-Coding RNA MALAT1 Enhances Ovarian Cancer Cell Stemness by Inhibiting YAP Translocation from Nucleus to Cytoplasm

Read this article at
Abstract
Background
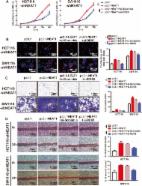
The purpose of this work was to unearth the effects and underlying mechanism of long non-coding RNA (lncRNA) MALAT1 in ovarian cancer cell stemness.
Material/Methods
Western blot, quantitative polymerase chain reaction (qPCR) and sphere forming analysis were performed to evaluate the stem-like traits of cells and MALAT1-induced effects on ovarian cancer cell stemness. Cell viability was performed to evaluate MALAT1 role in the chemoresistance of ovarian cancer cells. RNA immunoprecipitation (RIP) and luciferase reporter analysis were constructed to investigate the underlying mechanisms.
Results
Here, qPCR assay showed that MALAT1 level was remarkably higher in non-adherent spheres formed by adherent ovarian cancer cells, as well as cisplatin-resistant ovarian cancer cells. Additionally, MALAT1 knockdown reduced ovarian cancer cell stemness, characterized as the decrease of sphere forming ability, expression of stemness regulatory masters, and attenuation of cisplatin resistance. Moreover, MALAT1 interacted with yes-associated protein (YAP), inhibited its nuclear-cytoplasm translocation, promoted YAP protein stability and expression and thus increased its activity. Notably, rescuing expression of YAP attenuated the inhibition of MALAT1 knockdown on ovarian cancer cell stemness.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon.
- Record: found
- Abstract: found
- Article: found