- Record: found
- Abstract: found
- Article: found
The kallikrein–kinin pathway as a mechanism for auto-control of brown adipose tissue activity
Read this article at
Abstract
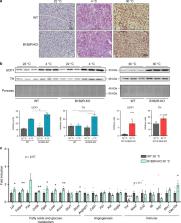
Brown adipose tissue (BAT) is known to secrete regulatory factors in response to thermogenic stimuli. Components of the BAT secretome may exert local effects that contribute to BAT recruitment and activation. Here, we found that a thermogenic stimulus leads to enhanced secretion of kininogen (Kng) by BAT, owing to induction of kininogen 2 ( Kng2) gene expression. Noradrenergic, cAMP-mediated signals induce KNG2 expression and release in brown adipocytes. Conversely, the expression of kinin receptors, that are activated by the Kng products bradykinin and [Des-Arg9]-bradykinin, are repressed by thermogenic activation of BAT in vivo and of brown adipocytes in vitro. Loss-of-function models for Kng (the circulating-Kng-deficient BN/Ka rat) and bradykinin (pharmacological inhibition of kinin receptors, kinin receptor-null mice) signaling were coincident in showing abnormal overactivation of BAT. Studies in vitro indicated that Kng and bradykinin exert repressive effects on brown adipocyte thermogenic activity by interfering the PKA/p38 MAPK pathway of control of Ucp1 gene transcription, whereas impaired kinin receptor expression enhances it. Our findings identify the kallikrein–kinin system as a relevant component of BAT thermogenic regulation that provides auto-regulatory inhibitory signaling to BAT.
Abstract
Brown adipose tissue, known produce heat by metabolizing fat, is also secretes molecules capable of communicating with other organs. Here the authors show that brown adipose tissue secretes kininogen, a component of heat system regulation, that provides auto-regulatory inhibitory signaling in brown adipose tissue.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Brown adipose tissue: function and physiological significance.
- Record: found
- Abstract: found
- Article: not found
Brown adipose tissue as a secretory organ.
- Record: found
- Abstract: found
- Article: not found