- Record: found
- Abstract: found
- Article: found
Single versus dual antiplatelet therapy following peripheral arterial endovascular intervention for chronic limb threatening ischaemia: Retrospective cohort study
Read this article at
Abstract
Objectives
Antiplatelet therapy following peripheral arterial endovascular intervention lacks high quality evidence to guide practice. The aim of this study was to assess the effect of three months of dual antiplatelet therapy on amputation-free survival following peripheral arterial endovascular intervention in patients with chronic limb threatening ischemia.
Methods
A retrospective review of symptomatic patients undergoing primary peripheral arterial endovascular intervention over a seven-year period was performed. The primary outcome measure was amputation-free survival. A sample size calculation based on previous cohort studies suggested that 629 limbs would be required to show a difference between single and dual therapy. Kaplan-Meier estimates and multivariate logistic regression analysis of recorded baseline characteristics was performed to determine predictors of amputation-free survival. Dual antiplatelet therapy was routinely given for 3 months.
Results
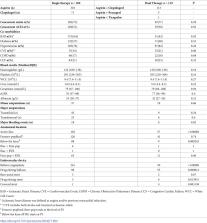
754 limbs were treated with primary angioplasty and/or stenting over a 7-year period, 508 of these for chronic limb threatening ischemia. There was no difference in unadjusted amputation-free survival between patients with chronic limb threatening ischaemia taking single vs. dual antiplatelet therapy (69% vs. 74% respectively Log rank Chi 2 = 0.1, p = .72). After adjusting for confounders, at 1 year there was also no significant difference in amputation-free survival between patients taking single vs. dual antiplatelet therapy [OR 0.8, 95% CI 0.5–1.2, p = .3]. There was no difference in rates of major bleeding between single and dual antiplatelet therapy.
Conclusions
There was no clear evidence of reduced amputation-free survival in patients with chronic limb threatening ischemia undergoing peripheral arterial endovascular intervention being treated with dual antiplatelet therapy for 3 months. This is at odds with other retrospective case series and highlights the limitations in basing clinical practice on such data. There is a need for an adequately powered, independent randomised trial to definitively answer the question.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia
- Record: found
- Abstract: not found
- Article: not found
2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS)

- Record: found
- Abstract: found
- Article: found