- Record: found
- Abstract: found
- Article: found
Longitudinal Maintenance of Cognitive Health in Centenarians in the 100-plus Study
Read this article at
Key Points
Findings
In this cohort study of 340 centenarians, most individuals who scored 26 or higher on the Mini-Mental State Examination at study inclusion maintained this level of performance for at least 2 years of follow-up, despite having risk factors associated with cognitive decline. This group represents less than 10% of the centenarians in the Dutch population.
Abstract
This cohort study of Dutch centenarians examines individuals who escape cognitive decline until extreme ages and investigates the prevalence of risk factors associated with cognitive decline.
Abstract
Importance
Some individuals who reach ages beyond 100 years in good cognitive health may be resilient against risk factors associated with cognitive decline. Exploring the processes underlying resilience may contribute to the development of therapeutic strategies that help to maintain cognitive health while aging.
Objective
To identify individuals who escape cognitive decline until extreme ages and to investigate the prevalence of associated risk factors.
Design, Setting, and Participants
The 100-plus Study is a prospective observational cohort study of community-based Dutch centenarians enrolled between 2013 and 2019 who were visited annually until death or until participation was no longer possible. The centenarians self-reported their cognitive health, as confirmed by a proxy. Of the 1023 centenarians approached for study inclusion, 340 fulfilled the study criteria and were included in analyses. Data analysis was performed from April 2019 to December 2019.
Main Outcomes and Measures
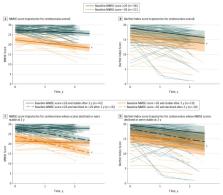
Cognition was assessed using the Mini-Mental State Examination (MMSE). To identify centenarians who escape cognitive decline, this study investigated the association of baseline cognition with survivorship and cognitive trajectories for at least 2 years of follow-up using linear mixed models, adjusted for sex, age, and education. This study investigated the prevalence of apolipoprotein E ( APOE) genotypes and cardiovascular disease as risk factors associated with cognitive decline.
Results
At baseline, the median age of 340 centenarians was 100.5 years (range, 100.0-108.2 years); 245 participants (72.1%) were female. The maximum survival estimate plateaued at 82% per year (95% CI, 77% to 87%) across centenarians who scored 26 to 30 points on the baseline MMSE (hazard ratio, 0.56; 95% CI, 0.42 to 0.75; P < .001), suggesting that an MMSE score of 26 or higher is representative of both cognitive and physical health. Among the 79 centenarians who were followed up for 2 years or longer, those with baseline MMSE score less than 26 experienced a decline in MMSE score of 1.68 points per year (95% CI, −2.45 to −0.92 points per year; P = .02), whereas centenarians with MMSE scores of 26 or higher at baseline experienced a decline of 0.71 point per year (95% CI, −1.08 to −0.35 points per year). For 73% of the centenarians with baseline MMSE scores of 26 or higher, no cognitive changes were observed, which often extended to ensuing years or until death. It is estimated that this group is representative of less than 10% of Dutch centenarians. In this group, 18.6% carried at least 1 APOE-ε4 allele, compared with 5.6% of the centenarians with lower and/or declining cognitive performance.
Conclusions and Relevance
Most centenarians who scored 26 or higher on the MMSE at baseline maintained high levels of cognitive performance for at least 2 years, in some cases despite the presence of risk factors associated with cognitive decline. Investigation of this group might reveal the processes underlying resilience against risk factors associated with cognitive decline.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: found
Resistance to autosomal dominant Alzheimer’s in an APOE3 -Christchurch homozygote: a case report
- Record: found
- Abstract: found
- Article: not found
A major role for cardiovascular burden in age-related cognitive decline.
- Record: found
- Abstract: found
- Article: not found