- Record: found
- Abstract: found
- Article: found
Targeting cellular metabolism to improve cancer therapeutics
Read this article at
Abstract
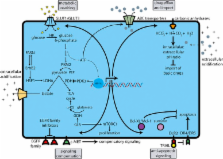
The metabolic properties of cancer cells diverge significantly from those of normal cells. Energy production in cancer cells is abnormally dependent on aerobic glycolysis. In addition to the dependency on glycolysis, cancer cells have other atypical metabolic characteristics such as increased fatty acid synthesis and increased rates of glutamine metabolism. Emerging evidence shows that many features characteristic to cancer cells, such as dysregulated Warburg-like glucose metabolism, fatty acid synthesis and glutaminolysis are linked to therapeutic resistance in cancer treatment. Therefore, targeting cellular metabolism may improve the response to cancer therapeutics and the combination of chemotherapeutic drugs with cellular metabolism inhibitors may represent a promising strategy to overcome drug resistance in cancer therapy. Recently, several review articles have summarized the anticancer targets in the metabolic pathways and metabolic inhibitor-induced cell death pathways, however, the dysregulated metabolism in therapeutic resistance, which is a highly clinical relevant area in cancer metabolism research, has not been specifically addressed. From this unique angle, this review article will discuss the relationship between dysregulated cellular metabolism and cancer drug resistance and how targeting of metabolic enzymes, such as glucose transporters, hexokinase, pyruvate kinase M2, lactate dehydrogenase A, pyruvate dehydrogenase kinase, fatty acid synthase and glutaminase can enhance the efficacy of common therapeutic agents or overcome resistance to chemotherapy or radiotherapy.
Related collections
Most cited references71
- Record: found
- Abstract: found
- Article: not found
Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia.
- Record: found
- Abstract: found
- Article: not found