- Record: found
- Abstract: found
- Article: found
Hypertrophy of human embryonic stem cell–derived cardiomyocytes supported by positive feedback between Ca 2+ and diacylglycerol signals
Read this article at
Abstract
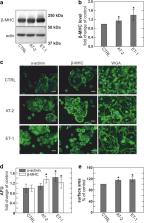
Human embryonic stem cell–derived cardiomyocytes develop pronounced hypertrophy in response to angiotensin-2, endothelin-1, and a selected mix of three fatty acids. All three of these responses are accompanied by increases in both basal cytoplasmic Ca 2+ and diacylglycerol, quantified with the Ca 2+ sensor Fluo-4 and a FRET-based diacylglycerol sensor expressed in these cardiomyocytes. The heart glycoside, ouabain (30 nM), and a recently developed inhibitor of diacylglycerol lipases, DO34 (1 μM), cause similar hypertrophy responses, and both responses are accompanied by equivalent increases of basal Ca 2+ and diacylglycerol. These results together suggest that basal Ca 2+ and diacylglycerol form a positive feedback signaling loop that promotes execution of cardiac growth programs in these human myocytes. Given that basal Ca 2+ in myocytes depends strongly on the Na + gradient, we also tested whether nanomolar ouabain concentrations might stimulate Na +/K + pumps, as described by others, and thereby prevent hypertrophy. However, stimulatory effects of nanomolar ouabain (1.5 nM) were not verified on Na +/K + pump currents in stem cell–derived myocytes, nor did nanomolar ouabain block hypertrophy induced by endothelin-1. Thus, low-dose ouabain is not a “protective” intervention under the conditions of these experiments in this human myocyte model. To summarize, the major aim of this study has been to characterize the progression of hypertrophy in human embryonic stem cell–derived cardiac myocytes in dependence on diacylglycerol and Na + gradient changes, developing a case that positive feedback coupling between these mechanisms plays an important role in the initiation of hypertrophy programs.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: not found
Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia.
- Record: found
- Abstract: found
- Article: not found
Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes.
- Record: found
- Abstract: found
- Article: not found