- Record: found
- Abstract: found
- Article: found
N-heteroatom substitution effect in 3-aza-cope rearrangements
Read this article at
Abstract
Background
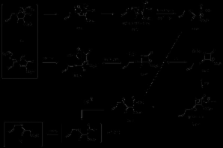
The nature of the heteroatom substitution in the nitrogen of a 3-aza-Cope system is explored.
Results
While N-propargyl isoxazolin-5-ones suffer 3-aza-Cope rearrangements at 60°C, the corresponding N-propargyl pyrazol-5-ones need a higher temperature of 180°C for the equivalent reaction. When the propargyl group is substituted by an allyl group, the temperature of the rearrangement for both type of compounds is less affected by the nature of the heteroatom present. Treatment with a base, such as ethoxide, facilitates the rearrangement, and in the case of isoxazol-5- ones other ring opening reactions take precedence, involving N–O ring cleavage of the 5-membered ring. However when base-catalysed decomposition is prevented by substituents, products arising from a room temperature aza-Cope rearrangement are isolated. A possible mechanistic pathway based on free energies derived from density functional calculations involving cyclic intermediates is proposed.
Related collections
Most cited references17
- Record: found
- Abstract: not found
- Article: not found
The Nicholas reaction: the use of dicobalt hexacarbonyl-stabilised propargylic cations in synthesis
- Record: found
- Abstract: found
- Article: not found
Aza Cope rearrangement of propargyl enammonium cations catalyzed by a self-assembled "nanozyme".
- Record: found
- Abstract: not found
- Article: not found