- Record: found
- Abstract: found
- Article: not found
Tissue level, activation and cellular localisation of TGF- β1 and association with survival in gastric cancer patients
Read this article at
Abstract
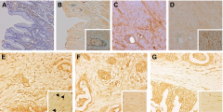
Transforming growth factor- β1 (TGF- β1), a tumour suppressing as well as tumour-promoting cytokine, is stored as an extracellular matrix-bound latent complex. We examined TGF- β1 activation and localisation of TGF- β1 activity in gastric cancer. Gastric tumours showed increased stromal and epithelial total TGF- β1 staining by immunohistochemistry. Active TGF- β1 was present in malignant epithelial cells, but most strongly in smooth muscle actin expressing fibroblasts. Normal gastric mucosa from the same patient showed some staining for total, and little for active TGF- β1. Active TGF- β1 levels were determined by ELISA on tissue homogenates, confirming a strong increase in active TGF- β1 in tumours compared to corresponding normal mucosa. Moreover, high tumour TGF- β1 activity levels were significantly associated with clinical parameters, including worse survival of the patients. Total and active TGF- β1 levels were not correlated, suggesting a specific activation process. Of the different proteases tested, active TGF- β1 levels were only correlated with urokinase activity levels. The correlation with urokinase activity suggests a role for plasmin in TGF- β1 activation in the tumour microenvironment, resulting in transformation of resident fibroblasts to tumour promoting myofibroblasts. In conclusion we have shown localisation and clinical relevance of TGF- β1 activity levels in gastric cancer.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Making sense of latent TGFbeta activation.
- Record: found
- Abstract: found
- Article: not found
