- Record: found
- Abstract: found
- Article: found
Diseases of complement dysregulation—an overview

Read this article at
Abstract
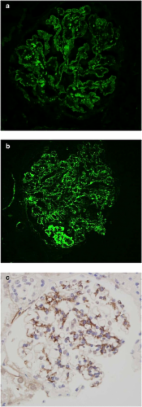
Atypical hemolytic uremic syndrome (aHUS), C3 glomerulopathy (C3G), and paroxysmal nocturnal hemoglobinuria (PNH) are prototypical disorders of complement dysregulation. Although complement overactivation is common to all, cell surface alternative pathway dysregulation (aHUS), fluid phase alternative pathway dysregulation (C3G), or terminal pathway dysregulation (PNH) predominates resulting in the very different phenotypes seen in these diseases. The mechanism underlying the dysregulation also varies with predominant acquired autoimmune (C3G), somatic mutations (PNH), or inherited germline mutations (aHUS) predisposing to disease. Eculizumab has revolutionized the treatment of PNH and aHUS although has been less successful in C3G. With the next generation of complement therapeutic in late stage development, these archetypal complement diseases will provide the initial targets.
Related collections
Most cited references146
- Record: found
- Abstract: found
- Article: not found
The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease.
- Record: found
- Abstract: found
- Article: not found
Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference.