- Record: found
- Abstract: found
- Article: not found
Augmented Stat5 Signaling Bypasses Multiple Impediments to Lactogen-Mediated Proliferation in Human β-Cells
Read this article at
Abstract
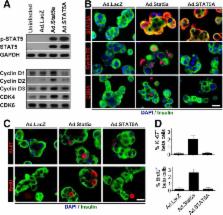
Pregnancy in rodents is associated with a two- to threefold increase in β-cell mass, which is attributable to large increases in β-cell proliferation, complimented by increases in β-cell size, survival, and function and mediated mainly by the lactogenic hormones prolactin (PRL) and placental lactogens. In humans, however, β-cell mass does not increase as dramatically during pregnancy, and PRL fails to activate proliferation in human islets in vitro. To determine why, we explored the human PRL–prolactin receptor (hPRLR)–Janus kinase 2 (JAK2)–signal transducer and activator of transcription 5 (STAT5)–cyclin–cdk signaling cascade in human β-cells. Surprisingly, adult human β-cells express little or no PRLR. As expected, restoration of the hPRLR in human β-cells rescued JAK2-STAT5 signaling in response to PRL. However, rescuing hPRLR-STAT5 signaling nevertheless failed to confer proliferative ability on adult human β-cells in response to PRL. Surprisingly, mouse (but not human) Stat5a overexpression led to upregulation of cyclins D1–3 and cdk4, as well as their nuclear translocation, all of which are associated with β-cell cycle entry. Collectively, the findings show that human β-cells fail to proliferate in response to PRL for multiple reasons, one of which is a paucity of functional PRL receptors, and that murine Stat5 overexpression is able to bypass these impediments.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Pancreatic beta-cell mass in European subjects with type 2 diabetes.
- Record: found
- Abstract: found
- Article: not found
Serotonin Regulates Pancreatic β-Cell Mass during Pregnancy
- Record: found
- Abstract: found
- Article: not found
