- Record: found
- Abstract: not found
- Article: not found
Continuous positive airway pressure (CPAP) as a ceiling of care treatment for hypoxemic respiratory failure due to COVID-19
research-article
Patrick Bradley
1 ,
Jennifer Nixon
2
,
,
James Wilson
1 ,
James Redfern
2 ,
Tarek Saba
1 ,
Emily Nuttall
2 ,
Thomas Bongers
1
22 February 2021
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Among patients admitted to hospital with COVID-19 in the UK, 10% develop severe hypoxemic
respiratory failure managed with invasive mechanical ventilation (IMV).
1
Much interest has focused on non-invasive strategies to avert progression to IMV.
UK guidelines recommend the use of continuous positive airway pressure (CPAP), including
in patients for whom IMV is not appropriate.
2
However, other nations have recommended against the use of CPAP,
3
and within the UK, CPAP use has varied widely (personal communication).
The greatest burden of COVID-19 disease is carried by older patients with comorbidities,
many of whom are deemed unsuitable for IMV and critical care. However, it is unclear
whether they might benefit from CPAP. The RECOVERY-RS trial is investigating the efficacy
of CPAP and high-flow nasal oxygen (HFNO) in severely hypoxic patients with COVID-19,
but will not complete until late 2021, and excludes patients unsuitable for IMV.
4
Current evidence is limited to cohort studies of heterogeneous patient groups, with
no published data focussing on patients for whom CPAP is the ceiling-of-care.
5
–7
Physicians caring for such patients, and those involved in planning the delivery of
CPAP services, must balance any potential benefits of CPAP against its burden on patients,
families, staff, and services. Therefore data in this patient population are urgently
needed.
Methods
We included all patients commenced on CPAP as a ceiling-of-care for treatment of respiratory
failure due to COVID-19, on the wards of two large UK hospitals. Data were collected
during the first 2 months of CPAP provision for COVID-19 at these sites, (26/3/20
– 25/5/20). Patients were excluded if they were suitable for escalation to IMV, or
received CPAP in a critical care setting.
Suitability for CPAP was based on local clinical guidelines; these recommended considering
CPAP if FiO2 ≥40% (site 1) or ≥35% (site 2) was required to maintain SpO2 ≥92% (or
88% if risk of CO2 retention). Guidelines at both sites recommended assessing clinical
frailty scale (CFS) to inform individualised decision making, with CFS ≤ 6 a suggested
threshold for treatment. CPAP was delivered using air-driven SleepCube (DeVilbiss)
and A40 (Philips Respironics) machines, with oxygen entrained to a face mask interface,
as per national guidance.
2
Data about patient and CPAP factors, prior to and during CPAP treatment, were collected.
Their relationship with 30-day mortality was tested for statistical significance using
standard non-parametric methods. Institutional approval was granted to conduct this
service evaluation, so research ethics approval was not required.
Results
70 patients were included; all were followed up to 30 days, or to hospital discharge
if this was beyond 30 days. The median age was 76 years [IQR 69–80], with median CFS
of 5 [IQR 4–6]. Initial CPAP settings used were pressure 5cmH2O [5–10] and O2 flow
10 L/min [10–15].
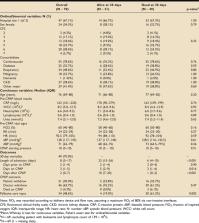
30 days after CPAP initiation, 21/70 (30%) patients were alive. Unadjusted comparison
of demographic and clinical variables between survivors and non-survivors showed no
statistically significant differences (Table 1). Changes in vital signs to 24 hours
after CPAP initiation also showed no significant differences (Figure 1).
Table 1.
Patient demographics, comorbidities, investigations, and outcomes.
Overall (N = 70)
Alive at 30 days (N = 21)
Dead at 30 days (N = 49)
p-valuea
Ordinal/binomial variables: N (%)
Hospital site 1 (of 2)
47 (67.1%)
14 (66.7%)
33 (67.3%)
1.00
Sex female
24 (34.3%)
8 (38.1%)
16 (32.7%)
0.79
CFS
2
3 (4.3%)
1 (4.8%)
2 (4.1%)
3
12 (17.1%)
4 (19.0%)
8 (16.3%)
4
13 (18.6%)
4 (19.0%)
9 (18.4%)
0.33
5
18 (25.7%)
2 (9.5%)
16 (32.7%)
6
20 (28.6%)
8 (38.1%)
12 (24.5%)
7
4 (5.7%)
2 (9.5%)
2 (4.1%)
Comorbidities
Cardiovascular
55 (78.6%)
16 (76.2%)
55 (78.6%)
0.76
Diabetes
25 (35.7%)
6 (28.6%)
19 (38.8%)
0.59
Respiratory
34 (48.6%)
11 (52.4%)
23 (46.9%)
0.80
Malignancy
18 (25.7%)
5 (23.8%)
13 (26.5%)
1.00
Dementia
1 (1.43%)
0 (0.0%)
1 (2.0%)
1.00
CKD
27 (38.6%)
8 (38.1%)
19 (38.8%)
1.00
Other major
29 (41.4%)
10 (47.6%)
19 (38.8%)
0.60
Continuous variables: Median (IQR)
Age (years)
76 (69–80)
71 (66–80)
77 (69–82)
0.20
Pre-CPAP blood results
CRP (mg/L)
162 (101–220)
170 (90–279)
162 (109–199)
0.74
WCC (109/L)a
8.2 (5.0–12.7)
8.2 (6.0–9.6)
8.4 (4.4–12.9)
0.92
Neutrophils (109/L)
6.6 (4.0–9.3)
7.1 (5.0–8.6)
6.0 (3.7–9.6)
0.77
Lymphocytes (109/L)a
0.6 (0.4–1.0)
0.6 (0.4–1.0)
0.6 (0.4–1.0)
0.89
Urea (mmol/L)
7.4 (5.1–12.0)
7.3 (4.6–12.0)
7.4 (5.2–11.4)
0.65
Pre-CPAP vital signs
FiO2 (%)
60 (40–80)
58 (40–60)
60 (50–80)
0.16
RR (/min)
24 (22–29)
24 (22–28)
24 (23–30)
0.27
HR (/min)
92.5 (79–105)
94 (84–110)
92 (78–104)
0.59
sBP (mmHg)b
128 (117–143)
127 (117–144)
128.5 (116–142.5)
0.61
dBP (mmHg)b
71 (62–79)
68 (62–75)
73 (64.5–79.5)
0.26
CPAP starting pressure
10 (5–10)
10 (5 – 10)
10 (5–10)
0.51
Outcomes
30-day mortality
49 (70.0%)
–
–
–
Length of admission (days)
8 (5–17)
23 (15–26)
6 (4–10)
<0.001
Days prior to CPAP
2 (1–4)
2 (1–4)
2 (0–4)
0.90
Days on CPAP
3 (1–5)
5 (2–9)
2 (1–4)
0.016
Days after CPAP
2 (0–7)
10 (7–20)
1 (0–2)
<0.001
CPAP outcome
Patient withdrew
21 (30.0%)
5 (23.8%)
16 (32.7%)
Doctor withdrew
46 (65.7%)
16 (76.2%)
30 (61.2%)
0.47
Died on CPAP
3 (4.3%)
0 (0.0%)
3 (6.1%)
Palliative care input
34 (48.6%)
4 (19.0%)
30 (61.2%)
0.002
Note: FiO2 was recorded according to delivery device and flow rate, assuming a maximum
FiO2 of 80% via non-invasive interfaces.
CFS: Rockwood clinical frailty scale; CKD: chronic kidney disease; CRP: C-reactive
protein; dBP: diastolic blood pressure; FiO2: fraction of inspired oxygen; IQR: interquartile
range; HR: heart rate; N: number; sBP: systolic blood pressure; WCC: white cell count.
aMann-Whitney U test for continuous variables, Fisher’s exact test for ordinal/binomial
variables.
aN = 69, excluding patient with leukaemia and lymphocyte count of 139 × 109/L.
bN = 69 (1 × missing data).
Figure 1.
Box and whisker plots showing trends in patient observations and CPAP settings as
percentage change per patient, with 2-hour CPAP values defined as baseline (matched).
Survivors spent a median 23 [15–26] days in hospital, with 5 [2–9] of those days on
CPAP. 3 patients (4.3%) died on CPAP and 21 (30%) chose to discontinue it; the remainder
discontinued CPAP according to clinical decisions (either weaning or futility).
Discussion
We evaluated two hospitals’ experience of offering CPAP as a ceiling-of-care treatment
for COVID-19. There was high mortality among this relatively frail cohort with a high
burden of comorbidity. Baseline patient characteristics were similar between survivors
and non-survivors. The notable rate of patient-initiated discontinuation of CPAP implies
a substantial burden of treatment. CPAP also prevents any visits from relatives, and
may potentially increase the risk of staff infection and emotional distress.
Limitations of this study include its relatively small sample size and lack of a control
group. However, this is among the largest reported cohorts of patients receiving CPAP
as a ceiling-of-care in this context, and the only one focussing solely on this group.
5
–7
Other strengths include the use of clinically relevant outcomes across two hospital
sites.
Given the ongoing widespread use of CPAP despite a lack of evidence, characterisation
of this patient group is urgently needed. Further work should include larger studies,
comparison with other management options, and exploration of the physical and psychological
effects of CPAP on patients and staff. While the efficacy of CPAP in this context
remains unproven, and its potential adverse effects unquantified, deciding whether,
and in whom, to offer it remains a matter for local protocols, clinical judgement,
and careful shared decision-making with patients.
Related collections
Most cited references1
- Record: found
- Abstract: found
- Article: found
Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicenter, cohort study
Stefano Aliberti, Dejan Radovanovic, Filippo Billi … (2020)
