- Record: found
- Abstract: found
- Article: found
4C3 Human Monoclonal Antibody: A Proof of Concept for Non-pathogenic Proteinase 3 Anti-neutrophil Cytoplasmic Antibodies in Granulomatosis With Polyangiitis
Read this article at
Abstract
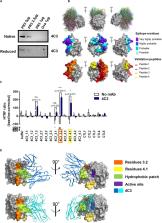
Granulomatosis with polyangiitis (GPA) is a severe autoimmune vasculitis associated with the presence of anti-neutrophil cytoplasmic antibodies (ANCA) mainly targeting proteinase 3 (PR3), a neutrophilic serine proteinase. PR3-ANCA binding to membrane-bound PR3 on neutrophils induce their auto-immune activation responsible for vascular lesions. However, the correlation between PR3-ANCA level and disease activity remains inconsistent, suggesting the existence of non-pathogenic PR3-ANCA. In order to prove their existence, we immortalized B lymphocytes from blood samples of GPA patients in remission having persistent PR3-ANCA to isolate non-activating PR3-ANCA. We obtained for the first time a non-activating human IgG1κ anti-PR3 monoclonal antibody (mAb) named 4C3. This new mAb binds soluble PR3 with a high affinity and membrane-bound PR3 on an epitope close to the PR3 hydrophobic patch and in the vicinity of the active site. 4C3 is able to bind FcγRIIA and FcγRIIIB and has a G2F glycosylation profile on asparagine 297. 4C3 did not induce activation of neutrophils and could inhibit human polyclonal PR3-ANCA-induced activation suggesting that 4C3 is non-pathogenic. This characteristic relies on the recognized epitope on PR3 rather than to the Fc portion properties. The existence of non-pathogenic PR3-ANCA, which do not activate neutrophils, could explain the persistence of high PR3-ANCA levels in some GPA patients in remission and why PR3-ANCA would not predict relapse. Finally, these results offer promising perspectives particularly regarding the understanding of PR3-ANCA pathogenicity and the development of new diagnostic and therapeutic strategies in GPA.
Related collections
Most cited references70
- Record: found
- Abstract: not found
- Article: not found
2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides.

- Record: found
- Abstract: found
- Article: found
IgG Subclasses and Allotypes: From Structure to Effector Functions
- Record: found
- Abstract: found
- Article: not found