- Record: found
- Abstract: found
- Article: found
Vaping Away Epithelial Integrity
editorial
August 2019
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Lung epithelial cells are the first line of defense against pathogens, chemicals,
and xenobiotics. With each breath, we inhale a plethora of foreign antigens that may
elicit a host immune response depending on the type of antigen or immunogen exposure,
genetic predisposition, and structural integrity of lung epithelial cells. The asserted
response also depends on the dose and chronicity of the exposure. The cellular complexity
of the airways and alveoli is astounding and includes ∼50 different cell types, ∼12
of which are epithelial (1). Almost half of these epithelial cells are ciliated (pseudostratified
columnar and cuboidal), with the remainder being comprised of goblet cells, basal
cells, club cells, and, at the terminal alveolus, type I and type II alveolar epithelial
cells (2). Together, these cells play a large and complex role in the host immune
responses to constant bombardment from foreign insults (inhaled or aspirated).
Epithelial injury initiates a variety of lung diseases, which may occur due to multiple
factors. Cigarette smoke is a major risk factor, particularly in chronic obstructive
pulmonary disease, asthma, and idiopathic pulmonary fibrosis. It is the principal
preventable cause of death and disease in the United States (3) and possibly worldwide.
With the growth in the use of e-cigarettes, also known as vaping, a current perception
is that these tobacco alternatives are safer than cigarettes. This concept has rapidly
led to an explosion in usage, especially among teens, who are attracted by the appealing
flavors and ease of use. However, the field lacks understanding of the mechanisms
related to the deleterious effects of e-cigarette vapor.
In this issue of the Journal, Lin and colleagues (pp. 162–173) report on an investigation
of the effects of e-cigarette vapor on CFTR (cystic fibrosis transmembrane conductance
regulator) in airway epithelial cells, and they describe a previously unknown dose-dependent
inhibitory effect of e-cigarette vapor on chloride anion transport by CFTR (4). Cigarette
smoke induces defects in CFTR function (5), but the effects of e-cigarettes were unknown.
Acquired dysfunction in CFTR resulting in impaired mucociliary transport and clearance
has previously been noted in patients with chronic obstructive pulmonary disease,
and especially in patients with chronic bronchitis (6, 7). Lin and colleagues establish
that the pyrolysis product of e-cigarettes, acrolein, reduces the short-circuit chloride
current without affecting cell survival. Toxic effects of e-cigarettes on airway epithelial
cells, including a reduction in their viability, were previously reported (8), although
the mechanisms remain elusive.
The authors also found that, unlike cigarette smoke, e-cigarette vapor reduces ion
conductance. The precise cause of this exclusive effect is not understood, but vaporization
appears to trigger the dysfunction. Lin and colleagues also showed that primary human
bronchial epithelial cells from donors were more sensitive to e-cigarette vapor-induced
inhibition of CFTR-dependent chloride transport than Calu-3 cells, perhaps due to
lower baseline expression of CFTR in human bronchial epithelial cells. This finding
is important as we consider different cell types and cell lines to study different
diseases. The authors detected a reduction in epithelial sodium channel activity,
which, in contrast to CFTR dysfunction, is reported with cigarette smoke as well.
Because CFTR also transports bicarbonate anions in addition to chloride anions, the
e-cigarette vapor-induced CFTR dysfunction might increase the pH on the apical surface
of airway epithelial cells and thus affect their physiology. The authors ruled out
any changes in the pH by checking the pH in basolateral media of cells exposed to
e-cigarette vapor. With longer exposure (60 min), they observed a reduction in transepithelial
electrical resistance with e-cigarette vapor, suggesting compromised barrier integrity.
Nonetheless, the precise mechanism and probable junctional proteins involved remain
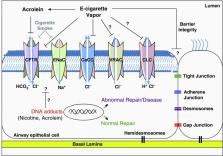
to be investigated (Figure 1).
Figure 1.
Potential mechanisms of e-cigarette vapor–induced damage to airway epithelium. CaCC
= calcium-activated chloride channel; CFTR = cystic fibrosis transmembrane conductance
regulator; CLC = chloride channel; ENaC = epithelial sodium channel; VRAC = volume-regulated
anion channel.
Because nicotine induces airway epithelial dysfunction by regulating CFTR function
through nicotinic acetylcholine receptors (9), it likely has similar effects when
inhaled as e-cigarette vapor. Like acrolein, nicotine has been associated with the
formation of DNA adducts (10). Apart from DNA damage, it also reduces XPC and 8-oxoguanine
DNA glycosylase 1/2 proteins, which are responsible for normal repair (11). The authors
alluded to potential effects of nicotine on CFTR, but focused only on acrolein in
this study. Likewise, other reactive aldehydes, reactive oxygen species (12), and
heavy metals were not investigated in this study, although these agents may have a
role in CFTR dysfunction in the airway epithelium (13). Acrolein has been shown to
directly modify CFTR and inhibit channel gating (5). Considering that nicotine and
acrolein form DNA adducts, it is plausible that they may directly or indirectly affect
a variety of ion channels (Figure 1).
Previous studies have shown that e-cigarettes dampen the ability of airway epithelial
cells to respond to viral infections, increase inflammation, and enhance pneumococcal
adherence (14–16). However, the precise mechanisms are not well understood. E-cigarette
products, with or without nicotine, have been shown to inhibit expression of SPLUNC1
(short palate, lung, and nasal epithelial clone 1), a molecule required for host defense
against human rhinovirus (15). These studies support the findings of Lin and colleagues,
and suggest that e-cigarettes may have far-reaching effects in addition to those of
nicotine or acrolein alone. Furthermore, other components present in e-cigarette vapor
need to be tested for their effect on airway epithelium. Broadly, the current study
by Lin and colleagues focuses attention on the involvement of ion channels in loss
of epithelial function and how this may lead to airway inflammation, infection, and
disease predisposition.
Although the authors observed attenuation of chloride current by CFTR dysfunction,
the question remains as to whether other chloride channels, such as CLC (17) and volume-regulated
chloride channels, are also affected (Figure 1). Lin and colleagues used primary human
bronchial epithelial cells from healthy donors to study the effects of e-cigarette
vapor. Further studies examining the effects of e-cigarette vapor on epithelial cells
derived from healthy versus diseased individuals might also provide information about
the functional changes that occur in these cells after exposure to e-cigarette vapor.
They may also address questions related to why some individuals do not develop lung
disease even after continuous exposure to e-cigarette vapor or cigarette smoke, whereas
others do. Moreover, incorporating animal studies will allow a fuller investigation
of the in vivo effects of e-cigarette vapor on lung cells and facilitate the use of
genetic manipulation of ion channels in airway epithelial cells that are affected
by e-cigarette vapor, and hence provide comprehensive methods to tease out the precise
mechanisms involved.
In summary, the present study provides novel insights into the mechanisms of airway
epithelial dysfunction caused by e-cigarette vapor and tobacco smoke through ion channels,
particularly CFTR. With evolving information about the chronic effects of e-cigarette
vapor on airway epithelium (18), these insights will improve our understanding of
how e-cigarettes affect cells and may help us devise targeted therapies against difficult
and progressive lung diseases.
Related collections
Most cited references17

- Record: found
- Abstract: found
- Article: found
E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells
Hyun-Wook Lee, Sung-Hyun Park, Mao-wen Weng … (2018)

- Record: found
- Abstract: found
- Article: found
Electronic Cigarette Liquid Increases Inflammation and Virus Infection in Primary Human Airway Epithelial Cells
Qun Wu, Di Jiang, Maisha Minor … (2014)
- Record: found
- Abstract: found
- Article: not found
Chronic E-Cigarette Exposure Alters the Human Bronchial Epithelial Proteome
Raymond C. Coakley, Arunava Ghosh, Keith Rogers … (2018)