- Record: found
- Abstract: found
- Article: found
Resolution of Inflammation: What Controls Its Onset?
Read this article at
Abstract
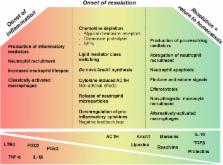
An effective resolution program may be able to prevent the progression from non-resolving acute inflammation to persistent chronic inflammation. It has now become evident that coordinated resolution programs initiate shortly after inflammatory responses begin. In this context, several mechanisms provide the fine-tuning of inflammation and create a favorable environment for the resolution phase to take place and for homeostasis to return. In this review, we focus on the events required for an effective transition from the proinflammatory phase to the onset and establishment of resolution. We suggest that several mediators that promote the inflammatory phase of inflammation can simultaneously initiate a program for active resolution. Indeed, several events enact a decrease in the local chemokine concentration, a reduction which is essential to inhibit further infiltration of neutrophils into the tissue. Interestingly, although neutrophils are cells that characteristically participate in the active phase of inflammation, they also contribute to the onset of resolution. Further understanding of the molecular mechanisms that initiate resolution may be instrumental to develop pro-resolution strategies to treat complex chronic inflammatory diseases, in humans. The efforts to develop strategies based on resolution of inflammation have shaped a new area of pharmacology referred to as “resolution pharmacology.”
Related collections
Most cited references198
- Record: found
- Abstract: found
- Article: not found
Origin and physiological roles of inflammation.
- Record: found
- Abstract: found
- Article: not found
Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production.
- Record: found
- Abstract: found
- Article: not found