- Record: found
- Abstract: found
- Article: not found
Glucose Sensing in L Cells: A Primary Cell Study
Read this article at
Summary
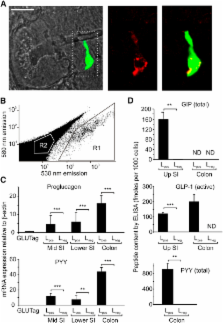
Glucagon-like peptide-1 (GLP-1) is an enteric hormone that stimulates insulin secretion and improves glycaemia in type 2 diabetes. Although GLP-1-based treatments are clinically available, alternative strategies to increase endogenous GLP-1 release from L cells are hampered by our limited physiological understanding of this cell type. By generating transgenic mice with L cell-specific expression of a fluorescent protein, we studied the characteristics of primary L cells by electrophysiology, fluorescence calcium imaging, and expression analysis and show that single L cells are electrically excitable and glucose responsive. Sensitivity to tolbutamide and low-millimolar concentrations of glucose and α-methylglucopyranoside, assessed in single L cells and by hormone secretion from primary cultures, suggested that GLP-1 release is regulated by the activity of sodium glucose cotransporter 1 and ATP-sensitive K + channels, consistent with their high expression levels in purified L cells by quantitative RT-PCR. These and other pathways identified using this approach will provide exciting opportunities for future physiological and therapeutic exploration.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1.
- Record: found
- Abstract: found
- Article: not found
T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1.
- Record: found
- Abstract: found
- Article: not found
