- Record: found
- Abstract: found
- Article: found
Message in a Microbottle: Modulation of Vascular Inflammation and Atherosclerosis by Extracellular Vesicles
Read this article at
Abstract
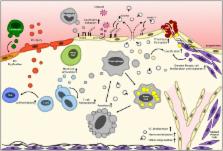
Extracellular vesicles (EVs) have emerged as a novel intercellular communication system. By carrying bioactive lipids, miRNAs and proteins they can modulate target cell functions and phenotype. Circulating levels of EVs are increased in inflammatory conditions, e.g., cardiovascular disease patients, and their functional contribution to atherosclerotic disease development is currently heavily studied. This review will describe how EVs can modulate vascular cell functions relevant to vascular inflammation and atherosclerosis, particularly highlighting the role of EV-associated proteolytic activity and effector proteins involved. Furthermore, we will discuss key questions and challenges, especially for EV-based therapeutics.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I.
- Record: found
- Abstract: found
- Article: not found
Cytokines in atherosclerosis: pathogenic and regulatory pathways.
- Record: found
- Abstract: found
- Article: not found