- Record: found
- Abstract: found
- Article: found
Heme as a Target for Therapeutic Interventions
Read this article at
Abstract
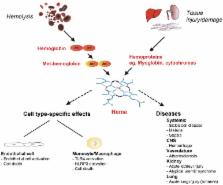
Heme is a complex of iron and the tetrapyrrole protoporphyrin IX with essential functions in aerobic organisms. Heme is the prosthetic group of hemoproteins such as hemoglobin and myoglobin, which are crucial for reversible oxygen binding and transport. By contrast, high levels of free heme, which may occur in various pathophysiological conditions, are toxic via pro-oxidant, pro-inflammatory and cytotoxic effects. The toxicity of heme plays a major role for the pathogenesis of prototypical hemolytic disorders including sickle cell disease and malaria. Moreover, there is increasing appreciation that detrimental effects of heme may also be critically involved in diseases, which usually are not associated with hemolysis such as severe sepsis and atherosclerosis. In mammalians homeostasis of heme and its potential toxicity are primarily controlled by two physiological systems. First, the scavenger protein hemopexin (Hx) non-covalently binds extracellular free heme with high affinity and attenuates toxicity of heme in plasma. Second, heme oxygenases (HOs), in particular the inducible HO isozyme, HO-1, can provide antioxidant cytoprotection via enzymatic degradation of intracellular heme. This review summarizes current knowledge on the pathophysiological role of heme for various diseases as demonstrated in experimental animal models and in humans. The functional significance of Hx and HOs for the regulation of heme homeostasis is highlighted. Finally, the therapeutic potential of pharmacological strategies that apply Hx and HO-1 in various clinical settings is discussed.
Related collections
Most cited references152
- Record: found
- Abstract: found
- Article: not found
Of mice and not men: differences between mouse and human immunology.
- Record: found
- Abstract: found
- Article: not found