- Record: found
- Abstract: found
- Article: not found
Touch and Tactile Neuropathic Pain Sensitivity Are Set by Corticospinal Projections
Abstract
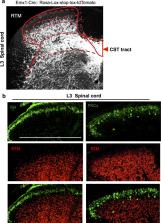
Current models of somatosensory perception emphasize transmission from primary sensory neurons to the spinal cord and on to the brain 1– 4 . Mental influence on perception is largely assumed to be acting locally within the brain. We have now examined if there is top-down control of sensory inflow through the spinal cord directly by the cortex. Although traditionally viewed as a primary motor pathway 5 , a subset of corticospinal neurons (CSNs) originating in the S1/S2 somatosensory cortex directly innervate the spinal dorsal horn via corticospinal tract (CST) axons. We show here that either reduction in somatosensory CSN activity or transection of the CST selectively impairs behavioral responses to light touch without altering responses to noxious stimuli. Moreover, such CSN manipulation greatly attenuates tactile allodynia in a peripheral neuropathic pain model. Tactile stimulation activates somatosensory CSNs and their corticospinal projections facilitate light touch-evoked activity of cholecystokinin (CCK +) interneurons in the deep dorsal horn. This represents a touch-driven feed forward spinal-cortical-spinal sensitization loop, which is important for the recruitment of spinal nociceptive neurons under tactile allodynia. These results reveal direct cortical modulation of normal and pathological tactile sensory processing in the spinal cord and open up opportunities for new treatments for neuropathic pain.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex.
- Record: found
- Abstract: found
- Article: not found
Spared nerve injury: an animal model of persistent peripheral neuropathic pain.
- Record: found
- Abstract: found
- Article: not found
