- Record: found
- Abstract: found
- Article: found
Expression and Role of the G Protein-Coupled Estrogen Receptor (GPR30/GPER) in the Development and Immune Response in Female Reproductive Cancers
Read this article at
Abstract
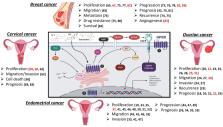
Cancer is a major public health issue and represents the second leading cause of death in women worldwide, as female reproductive-related neoplasms are the main cause of incidence and mortality. Female reproductive cancers have a close relationship to estrogens, the principal female sex steroid hormones. Estrogens exert their actions by the nuclear estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). ERα, and ERβ act as transcription factors mediating genomic effects. Besides, the G protein-coupled estrogen receptor (GPER, formerly known as GPR30) was recently described as a seven-transmembrane receptor that mediates non-genomic estrogenic signaling, including calcium mobilization, cAMP synthesis, cleavage of matrix metalloproteinases, transactivation of epidermal growth factor receptor (EGFR), and the subsequent activation of PI3K and MAPK signaling pathways, which are the reasons why it is related to cellular processes, such as cell-cycle progression, cellular proliferation, differentiation, apoptosis, migration, and invasion. Since its discovery, selective agonists and antagonists have been found and developed. GPER has been implicated in a variety of hormone-responsiveness tumors, such as breast, endometrial, ovarian, cervical, prostate, and testicular cancer as well as lung, hepatic, thyroid, colorectal, and adrenocortical cancers. Nevertheless, GPER actions in cancer are still debatable due to the conflicting information that has been reported to date, since many reports indicate that activation of this receptor can modulate carcinogenesis. In contrast, many others show that its activation inhibits tumor activity. Besides, estrogens play an essential role in the regulation of the immune system, but little information exists about the role of GPER activation on its modulation within cancer context. This review focuses on the role that the stimulation of GPER plays in female reproductive neoplasms, specifically breast, endometrial, ovarian, and cervical cancers, in its tumor activity and immune response regulation.
Related collections
Most cited references91
- Record: found
- Abstract: found
- Article: not found
Virtual and biomolecular screening converge on a selective agonist for GPR30.
- Record: found
- Abstract: found
- Article: not found