- Record: found
- Abstract: found
- Article: found
Anti-Inflammatory Effects of Progesterone in Lipopolysaccharide-Stimulated BV-2 Microglia
Read this article at
Abstract
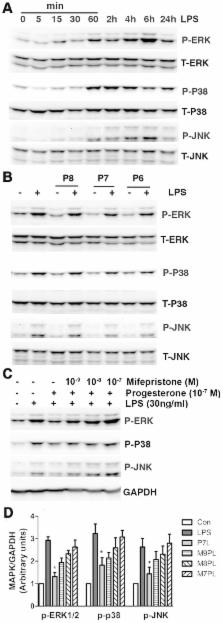
Female sex is associated with improved outcome in experimental brain injury models, such as traumatic brain injury, ischemic stroke, and intracerebral hemorrhage. This implies female gonadal steroids may be neuroprotective. A mechanism for this may involve modulation of post-injury neuroinflammation. As the resident immunomodulatory cells in central nervous system, microglia are activated during acute brain injury and produce inflammatory mediators which contribute to secondary injury including proinflammatory cytokines, and nitric oxide (NO) and prostaglandin E 2 (PGE 2), mediated by inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively. We hypothesized that female gonadal steroids reduce microglia mediated neuroinflammation. In this study, the progesterone’s effects on tumor necrosis factor alpha (TNF-α), iNOS, and COX-2 expression were investigated in lipopolysaccharide (LPS)-stimulated BV-2 microglia. Further, investigation included nuclear factor kappa B (NF-κB) and mitogen activated protein kinase (MAPK) pathways. LPS (30 ng/ml) upregulated TNF-α, iNOS, and COX-2 protein expression in BV-2 cells. Progesterone pretreatment attenuated LPS-stimulated TNF-α, iNOS, and COX-2 expression in a dose-dependent fashion. Progesterone suppressed LPS-induced NF-κB activation by decreasing inhibitory κBα and NF-κB p65 phosphorylation and p65 nuclear translocation. Progesterone decreased LPS-mediated phosphorylation of p38, c-Jun N-terminal kinase and extracellular regulated kinase MAPKs. These progesterone effects were inhibited by its antagonist mifepristone. In conclusion, progesterone exhibits pleiotropic anti-inflammatory effects in LPS-stimulated BV-2 microglia by down-regulating proinflammatory mediators corresponding to suppression of NF-κB and MAPK activation. This suggests progesterone may be used as a potential neurotherapeutic to treat inflammatory components of acute brain injury.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation.
- Record: found
- Abstract: found
- Article: not found
Caspase signalling controls microglia activation and neurotoxicity.
- Record: found
- Abstract: found
- Article: not found